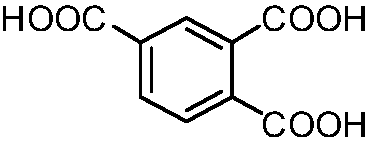
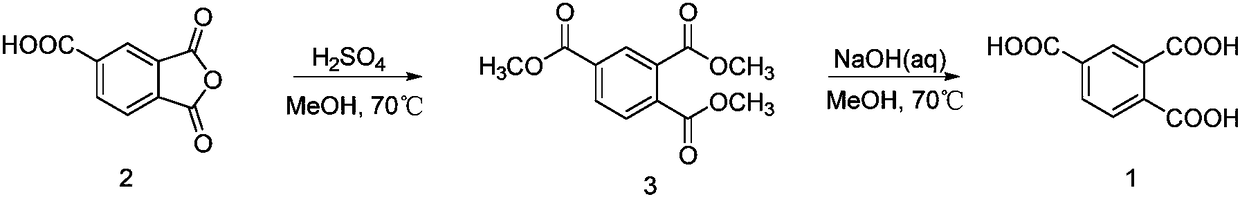
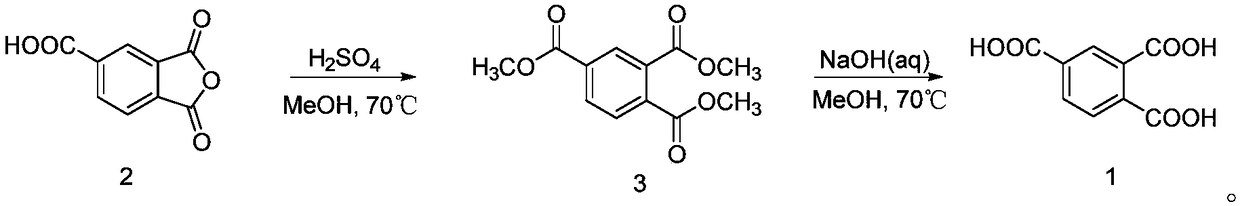
Method for synthesizing trimellitic acid
A trimellitic acid and synthesis method technology, applied in the field of trimellitic acid synthesis, can solve the problems of difficult reaction monitoring, harsh reaction conditions, incomplete reaction, etc., achieve cheap and easy reagents, reduce reaction time, and easy monitoring Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of Trimethyl 1,2,4-Benzenetricarboxylate
[0025] Add trimellitic anhydride (100.0 g (gram), 520.48 mmol (mmol)) to a 1 L (liter) round bottom flask, then add 500 mL of methanol to dissolve it, add 16 mL of concentrated sulfuric acid with a mass fraction of 98% dropwise, and heat at 70° C. Refluxing reaction, after 48h (hours), detected by TLC (thin layer chromatography), the reaction is over. Spin off methanol under reduced pressure at 50°C, add 200mL of water, extract with dichloromethane (30mL×10), combine the organic phases, wash with saturated sodium bicarbonate (100mL×3), wash with saturated brine (100mL×3), and finally After drying with anhydrous sodium sulfate and spin-drying, 118.4 g of colorless oily substance 1,2,4-benzenetricarboxylate trimethyl was obtained, with a yield of 90.20%.
[0026] 1 H NMR (400MHz, CDCl 3 )δ: 8.43(s, 1H), 8.21(d, J=8.0Hz, 1H), 7.76(d, J=8.0Hz, 1H), 3.96(s, 3H), 3.94(s, 6H); 13 C NMR (300MHz, CDCl3) δ: 167.63, 166.83, ...
Embodiment 2
[0031] In step (1), the mass volume ratio of trimellitic anhydride, methanol, and concentrated sulfuric acid is 1:4.5:0.15, the reaction temperature is 75°C, and the reaction time is 50 hours. The yield of trimethyl 1,2,4-benzenetricarboxylate is 89.88%.
[0032] The mass volume ratio of 1,2,4-benzenetricarboxylic acid trimethyl ester, methanol and sodium hydroxide in step (2): 1:4.5:3.0, the reaction temperature is 75°C, the reaction time is 40h, the yield of trimellitic acid: 90.02%.
Embodiment 3
[0034] In step (1), the mass volume ratio of trimellitic anhydride, methanol, and concentrated sulfuric acid is 1:5.5:0.18, the reaction temperature is 68°C, the reaction time is 65 hours, and the yield of trimethyl 1,2,4-benzenetricarboxylate is 89.92%.
[0035] Mass volume ratio of 1,2,4-benzenetricarboxylate, methanol and sodium hydroxide in step (2): 1:5.5:3.5, reaction temperature 68°C, reaction time 45h, yield of trimellitic acid: 89.78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com