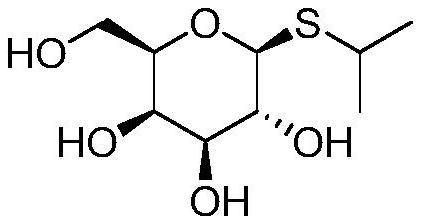
A kind of synthetic method of isopropyl-beta-d-thiogalactoside
A technology of thiogalactoside and synthesis method, which is applied in the field of synthesis of isopropyl-β-D-thiogalactoside, can solve the problems of increasing production costs and long steps, and achieve the avoidance of environmental hazards, The effect of low cost and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
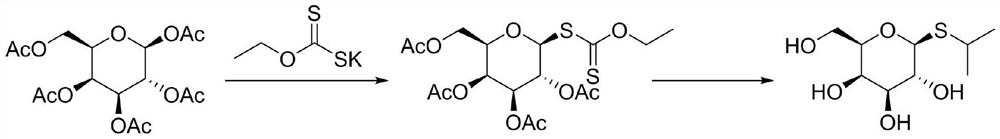
[0025] The first step, β-D-galactose pentaacetate (390.3 grams, 1mol) was dissolved in dichloromethane (1L), added boron trifluoride ether (5.7 grams, 0.04mol) and ethyl xanthic acid Potassium (160.3 g, 1 mol) was reacted, stirred and reacted for 3 hours at -5 ° C, the reaction was completed, the reaction solution was washed twice, and the dichloromethane was dried with anhydrous sodium sulfate. Then, the dichloromethane was removed by rotary evaporation to obtain Tetraacetyl galactose ethyl xanthate 420 grams, yield 93%. m / z=453.10(M+1);
[0026] Second step, get the above-mentioned tetraacetylsemisugar ethyl xanthate (226 grams, 0.5mol) and 2-bromopropane (67.7 grams, 0.55mol) and potassium carbonate (83 grams, 0.6mol) and be dissolved in 2L methanol reaction at 50°C, after 4 hours, the reaction is complete, add concentrated hydrochloric acid to adjust the pH value to 5-7, remove the potassium bromide generated during the reaction by suction filtration, and then remove the ...
Embodiment 2
[0028] The first step, β-D-galactose pentaacetate (390.3 grams, 1mol) was dissolved in chloroform (1.5L), added boron trifluoride ether (7 grams, 0.05mol) and ethyl xanthogen Potassium chloride (192.4 g, 1.2 mol) was reacted, stirred and reacted at -8°C for 4 hours, the reaction was completed, the reaction solution was washed twice with water, and the chloroform was dried with anhydrous sodium sulfate, and then the chloroform was removed by rotary evaporation. 428 g of tetraacetyl galactosyl xanthate was obtained with a yield of 95%.
[0029] The second step, get the above-mentioned tetraacetylgalactose ethyl xanthate (226 grams, 0.5mol) and 2-bromopropane (74 grams, 0.6mol) and potassium carbonate (90 grams, 0.65mol) and be dissolved in 2L methanol , react at 60°C, after 5 hours, the reaction is complete, add concentrated hydrochloric acid to adjust the pH value to 5-7, remove the potassium bromide generated during the reaction by suction filtration, and then remove the metha...
Embodiment 3
[0031] In the first step, β-D-galactose pentaacetate (390.3 grams, 1mol) was dissolved in methylene chloride (2L), and zinc chloride (5.5 grams, 0.04mol) and sodium ethyl xanthate ( 158.6 grams, 1.1mol) reacted, stirred and reacted at -3°C for 3 hours, the reaction was completed, the reaction solution was washed twice with water, and the dichloromethane was dried with anhydrous sodium sulfate. Then, the dichloromethane was removed by rotary evaporation to obtain tetraacetyl 412 grams of galactose ethyl xanthate, yield 91%.
[0032] The second step, get the above-mentioned tetraacetylgalactose ethyl xanthate (226 grams, 0.5mol) and 2-bromopropane (74 grams, 0.6mol) and sodium carbonate (80 grams, 0.75mol) and be dissolved in 2L ethanol , react at 70°C, after 5 hours, the reaction is complete, add concentrated hydrochloric acid to adjust the pH value to 5-7, remove the potassium bromide generated during the reaction by suction filtration, and then remove ethanol by rotary evapor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com