A kind of pyrrole-rhodamine acylhydrazone derivative and its preparation method and application
A technology of cyanohydrazone and derivatives, which is applied in the field of pyrrole-rhodamine acylhydrazone derivatives and its preparation, can solve the problem of few fluorescent probes, achieve high selectivity ratio fluorescence recognition performance, wide potential application value, The effect of easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of pyrrole-rhodamine acylhydrazone derivatives:
[0036]
[0037] N-(2-morpholine-4-ethyl)-5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxamide (279 mg, 1 mmol) was added to rhodamine 6G hydrazide ( 428mg, 1mmol) of absolute ethanol (30mL), heated to reflux for 3 hours, then cooled to room temperature, after cooling to room temperature, vacuum filtration under reduced pressure, washed filter cake with absolute ethanol, and then dried in air to obtain The white solid is the target product, and the yield is 72%.
[0038] The crystal structure of the pyrrole-rhodamine acylhydrazone derivative ethanolate was determined by single crystal diffraction, seefigure 1 .
[0039] Carry out nuclear magnetic resonance analysis to the obtained pyrrole-rhodamine acylhydrazone derivatives, see the specific nuclear magnetic spectrum figure 2 , figure 2 The analysis results are as follows:
[0040] 1 H NMR (400MHz, DMSO-d 6 ), δ (ppm): 11.09 (s, 1H, NH-pyrrole), 8.00 (s, 1H...
Embodiment 2
[0043] Determination of Optical Properties of Pyrrole-Rhodamine Hydrazone Derivatives to Divalent Copper Ions
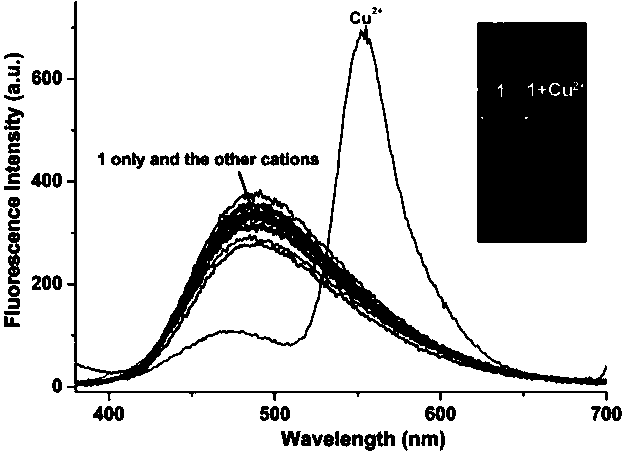
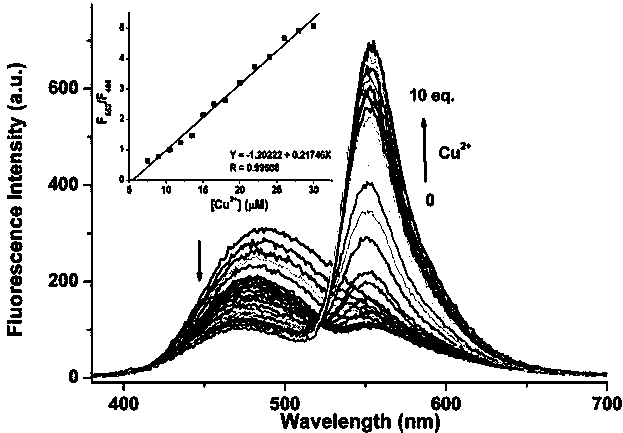
[0044] The pyrrole-rhodamine acylhydrazone derivatives prepared in the above example 1 were used as fluorescent probes in CH 3 CN / HEPES (volume ratio 8︰2) medium to prepare a molar concentration of 1×10 -5 mol / L solution, respectively, at a molar concentration of 3×10 -5 mol / L Ag + , Al 3+ , Ca 2+ , Cd 2+ ,Co 2+ , Cr 3+ , Cu 2+ , Fe 3+ , Hg 2+ , K + , Mg 2+ , Mn 2+ , Na + , Ni 2+ , Pb 2 and Zn 2+ Add an equal volume of the above-mentioned fluorescent probe solution to the aqueous solution of metal ions, and use a fluorescence spectrometer to perform fluorescence spectrum analysis (excitation wavelength is 360 nm, real-time detection), and the obtained fluorescence spectrum diagram is shown in Figure 4 . pass Figure 4 It can be seen that after the pyrrole-rhodamine acylhydrazone derivative prepared in Example 1 of the present invention acts as a ...
Embodiment 3
[0047] Detection experiment of pyrrole-rhodamine acylhydrazone derivatives in intracellular copper ions
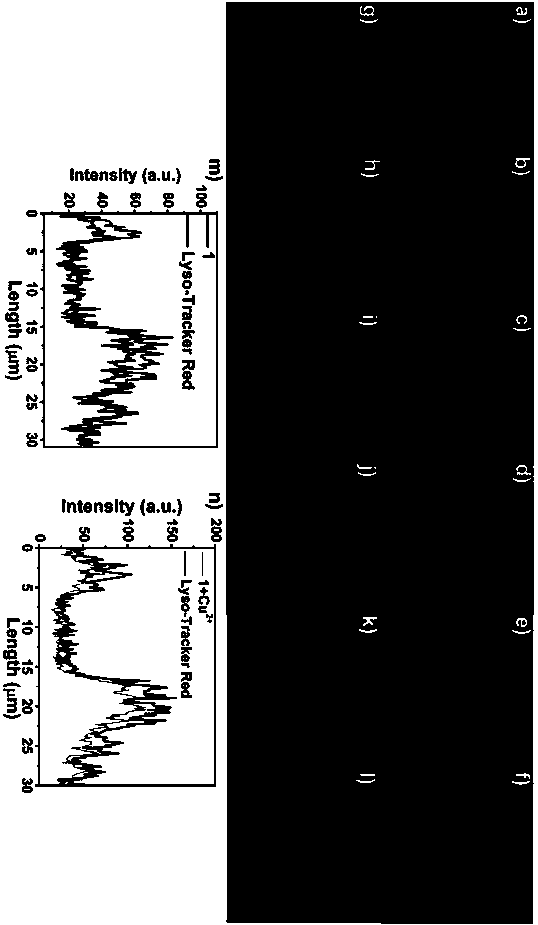
[0048] 1 x 10 for Hela cells -5 The pyrrole-rhodamine acylhydrazone derivative fluorescent probe prepared in the above-mentioned Example 2 of mol / L was incubated with commercial lysosome localization dye LysoTracker Red for 30 minutes and then added 2×10 -5 mol / L Cu 2+ solution (solvent is water) such that Cu 2+ The final concentration was 50 μmol / L, and after continuing to incubate for 30 minutes, use the Olympus FV500-IX70 laser confocal microscope to perform fluorescence imaging to obtain the fluorescence imaging map of Hela cells, specifically as follows Figure 6 shown. Fluorescent probe + LysoTracker Red co-staining: a is the fluorescence imaging image of the blue channel; b is the fluorescence imaging image of the green channel; c is the fluorescence imaging image of the red channel of LysoTracker Red; d is the overlay image of the blue channel, green channel a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com