Boron-containing heterocyclic compound and application thereof in organic photoelectric device
A technology of compound and boron heterocycle, applied in the field of boron-containing heterocycle compound and its application in organic optoelectronic devices, to achieve high thermal stability, low voltage, and improve the effect of luminescence stability
- Summary
- Abstract
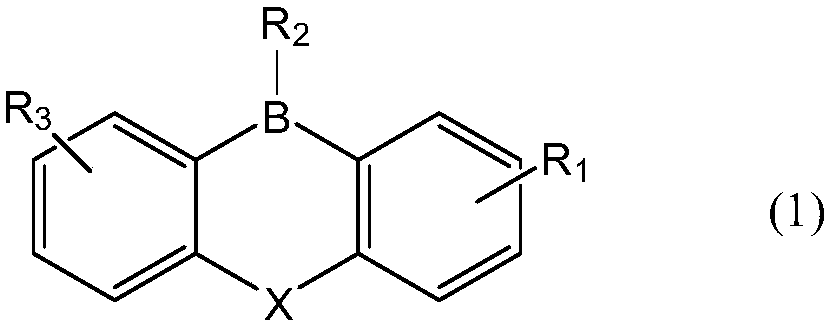
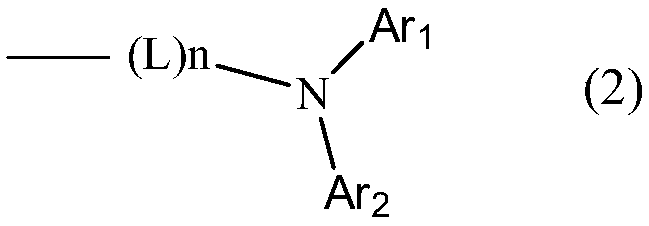
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
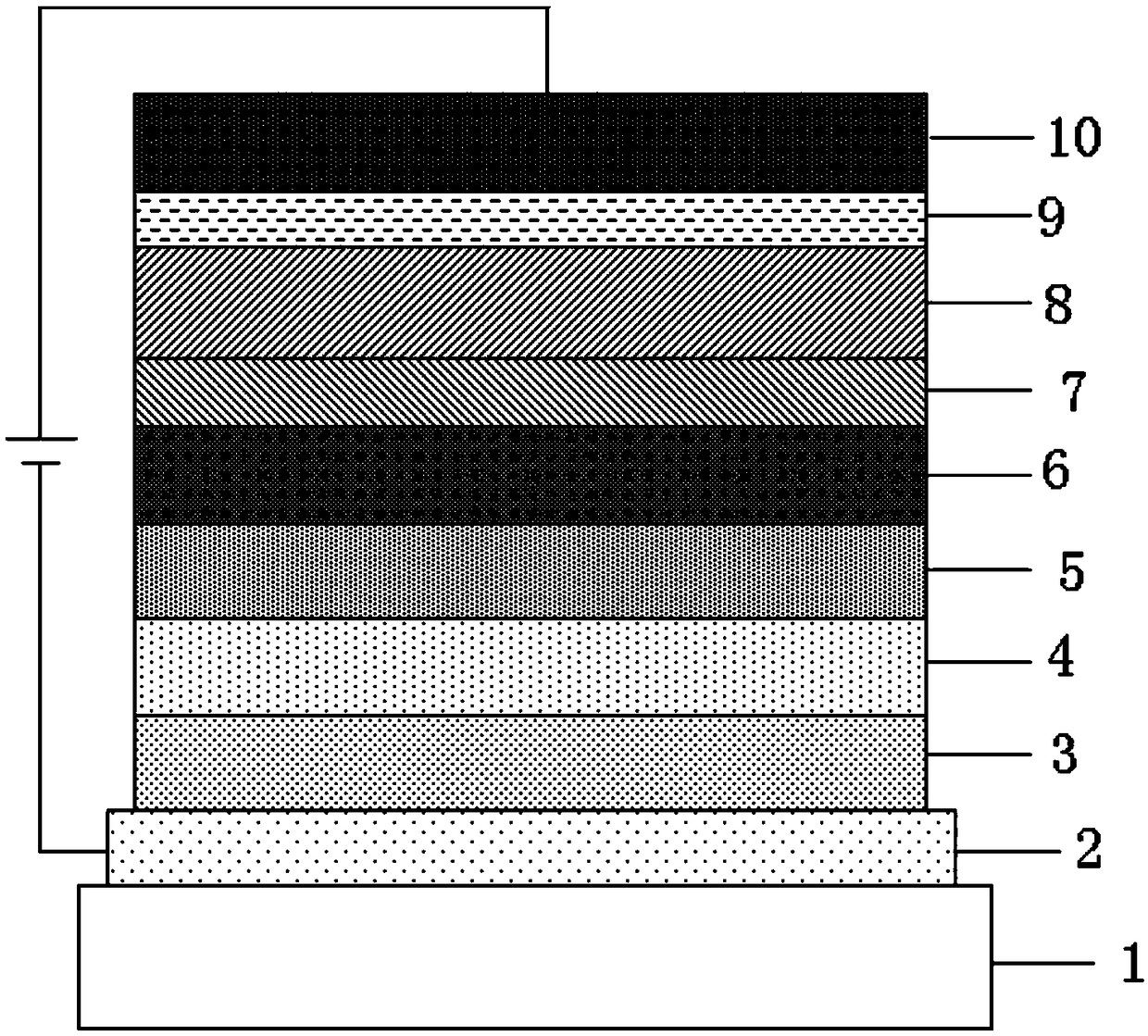
Image
Examples
Embodiment 1
[0112]
[0113] Add 25g of compound 3-1, 25g of 2,4-dibromo-1-fluoro-benzene, 300ml of dimethyl sulfoxide (DMSO) into a 500ml three-necked flask, blow nitrogen, stir to dissolve the raw materials completely, add K 2 CO 3 16.5g, the solution was yellow. Turn on the heating to 110°C for 8 hours and then take samples for monitoring. After the raw materials are completely reacted, cool down to room temperature for post-processing.
[0114] The reaction solution was added to 3 times the volume of water, the product was stirred to precipitate, filtered, the filter cake was dissolved in toluene, and washed with water until neutral. Anhydrous Na 2 SO 4 After drying for 2 hours, the desiccant was removed by filtration, and the filter cake was rinsed with a small amount of toluene. The filtrates were combined, concentrated and purified by column to obtain the target compound 3-2 as a yellow solid 36.1 g, with a yield of 76.2%.
[0115] 1 H NMR (400MHz, CDC13) δ7.61 (s, 2H), 7.3...
Embodiment 2
[0117]
[0118] Add 36g of compound 3-2 and 300ml of tetrahydrofuran (THF) into a 1L three-necked flask, replace the air in the reaction flask with nitrogen, cool down to -78°C, and slowly add n-butyllithium (n-BuLi) dropwise under nitrogen protection ( 2.0M in THF) 75ml, heat preservation reaction at this temperature for 2h, add 9.6g of phenylboronic acid, keep this temperature for 6h and then naturally warm up to room temperature, when TLC monitors that the reaction of raw materials is complete, add 1M ammonium chloride solution to adjust the pH to neutral After stirring for 10 minutes, let it stand for liquid separation. Anhydrous Na for organic phase 2 SO 4 After drying for 2 hours and filtering, the filtrate was concentrated and passed through a silica gel column to obtain 20.1 g of compound 3-3 as a white solid, with a yield of 65.3%.
[0119] 1 H NMR (400MHz, CDC13) δ7.42(t, J=7.6, 1H), 7.35(t, J=7.6, 2H), 7.31(d, J=7.6, 2H), 7.22-7.24(m, 4H) , 7.18 (d, J=7.6, 2H...
Embodiment 3
[0121]
[0122] Add 20g of compound 3-3, 200ml of glacial acetic acid (AcOH), 13g of 30% hydrogen peroxide in a 500ml three-necked flask, stir and react at room temperature for 2h, and after TLC monitors that the raw materials have reacted completely, add 1M sodium bicarbonate solution to adjust the pH to be neutral. After stirring for 10 min, let it stand for liquid separation. The aqueous phase was extracted with dichloromethane, and the combined organic phases were washed with anhydrous Na 2 SO 4 After drying for 2 hours and filtering, the filtrate was concentrated and passed through a silica gel column to obtain 20.1 g of compound 3-4 as a white solid, with a yield of 93.5%.
[0123] 1 H NMR (400MHz, CDC13) δ7.82(d, J=8.0, 2H), 7.66-7.73(m, 4H), 7.42(t, J=7.6, 1H), 7.35(t, J=7.6, 2H) , 7.31 (d, J=7.6, 2H);
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com