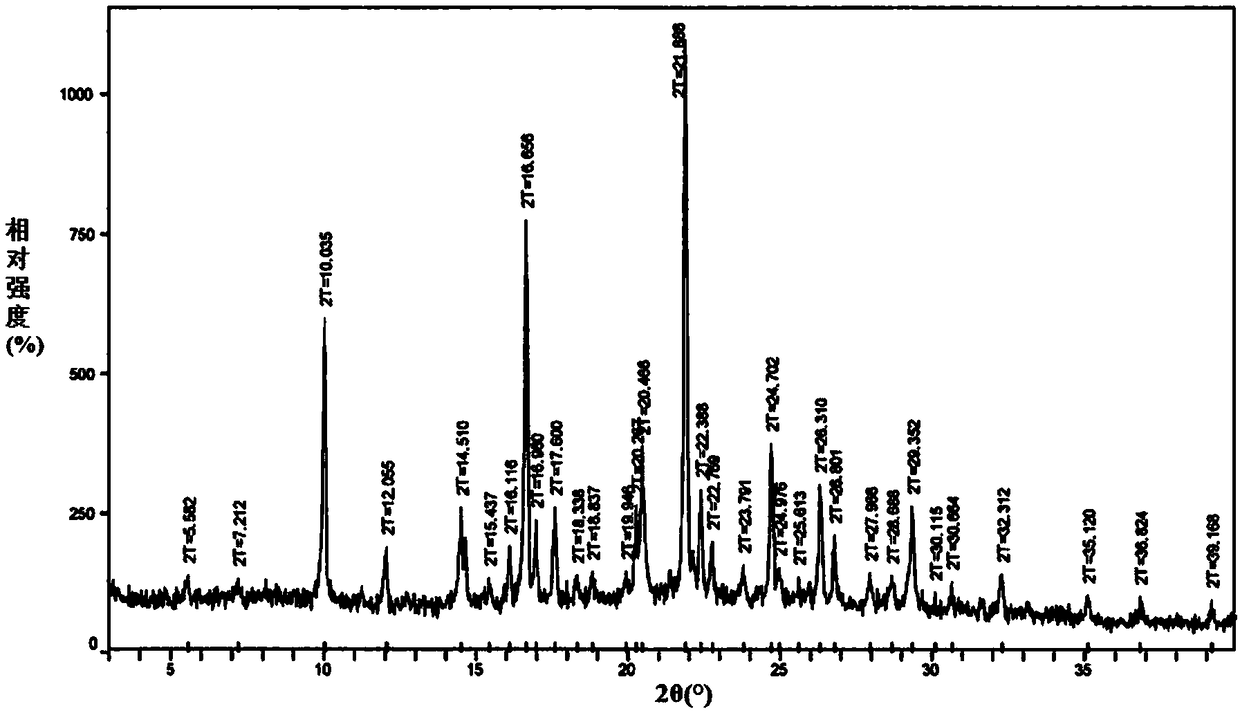
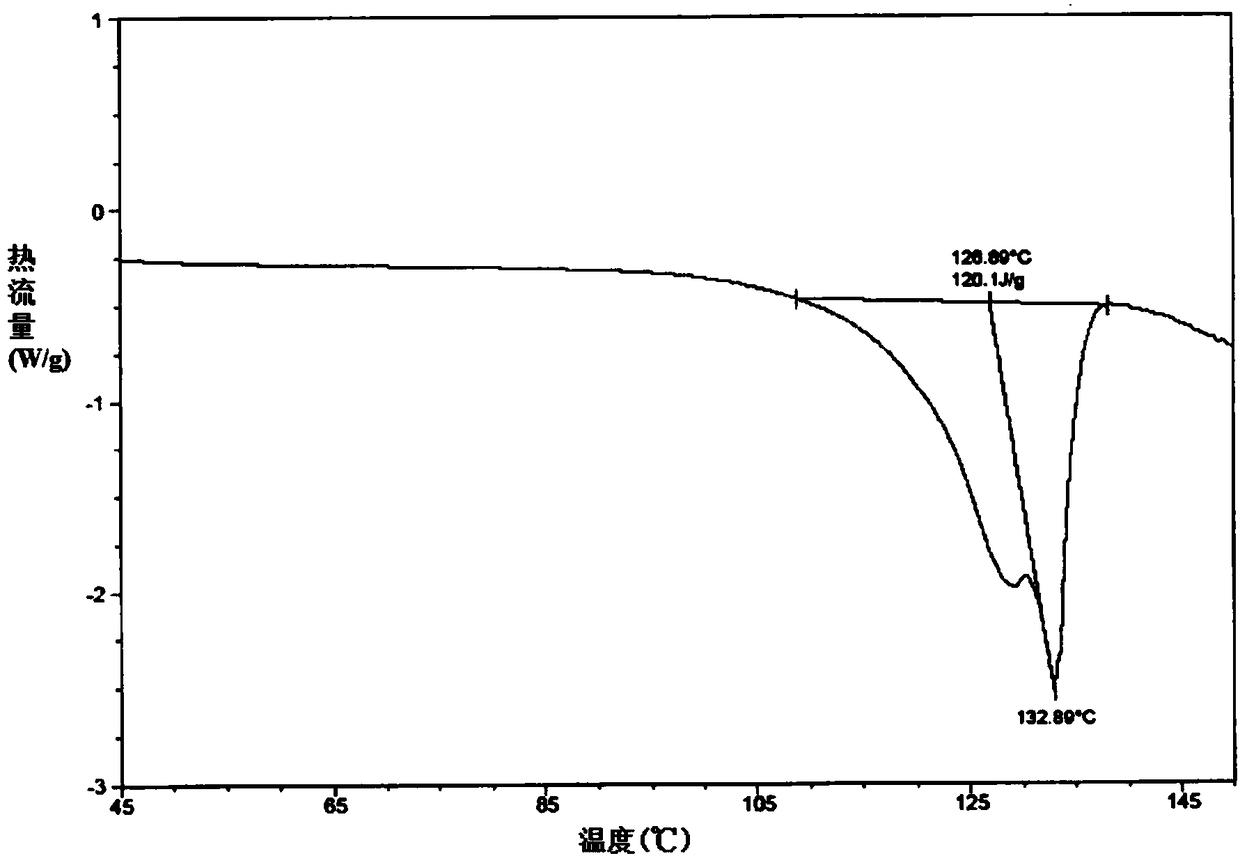
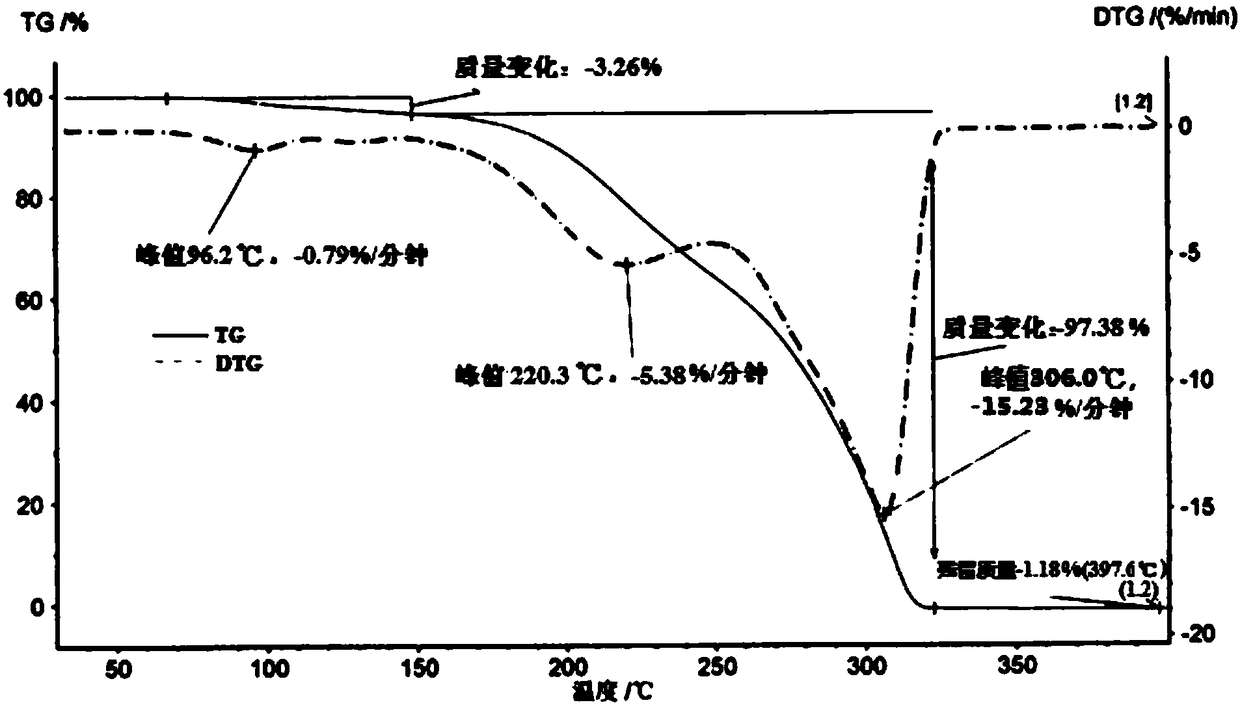
Ammonium carboxylate salt compound and crystal forms and amorphous substance thereof and preparation methods of compound, crystal forms and amorphous substance
A technology of compounds and crystal forms, applied in the preparation of organic compounds, the preparation of carboxylic acid amides, the preparation of cyanide reactions, etc., can solve problems such as difficulty in operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] Synthesis of embodiment 1 formula (A) compound
[0176] 1.1 Synthesis of (2R,4S)-5-([1,1-biphenyl)-4-amino-2-methylpentanoic acid ethyl ester hydrochloride:
[0177]
[0178] Add (2R,4S)-5-(biphenyl-4-yl)-4-[(tert-butoxycarbonyl)amino]-2-methylpentanoic acid ( 50g, 0.13mol) and absolute ethanol (500ml), stirred and dissolved at room temperature, and thionyl chloride (23.3g, 0.195mol) was added dropwise. After the addition, the temperature was raised to 50-60°C for 3-3.5h. Cool and concentrate to dryness under reduced pressure. The residue was washed with ethyl acetate, and the ethanol residue in the concentrate was monitored by GC to be no more than 15%, then ethyl acetate (500ml) was added, stirred at room temperature, and beaten for 3h; filtered and dried to obtain (2R,4S)-5-([1 , 1-biphenyl)-4-amino-2-methylpentanoic acid ethyl ester hydrochloride, the yield ranges from 88% to 96%, and the purity is greater than 98.5%.
[0179] 1.2 Synthesis of (2R,4S)-5-(biph...
Embodiment 2
[0185] Example 2 4(((2S,4R)-1-([1,1'-biphenyl]-4-yl)-5-ethoxy-4-methyl-5-oxopentane-2- Base) amino)-4-oxobutanoic acid ammonium synthesis:
[0186]
[0187] Add (2R,4S)-5-(biphenyl-4-yl)-4-[(3-carboxypropionyl)amino prepared according to the method of step 1.2 of Example 1 into a 1L three-necked flask equipped with mechanical stirring ]-2-methylpentanoic acid ethyl ester crude product (60.0g, 0.145mol) and acetone (720ml), stirring and dissolving at room temperature; cooling to 0-10°C for 5-10min, dropwise adding concentrated ammonia water (24.4g, 0.358mol), dropwise After the addition, continue to stir for 4h, filter, wash the filter cake with acetone (72ml), 10-30°C, -0.09~-0.1MPa, and vacuum-dry for 4-6h; 46.86g of solid 4-(((2S,4R)- Ammonium 1-([1,1'-biphenyl]-4-yl)-5-ethoxy-4-methyl-5-oxopentan-2-yl)amino)-4-oxobutanoate , that is, the compound of formula (A), the yield ranges from 70% to 75%, the purity is greater than 99.5%, the process repeatability is higher than...
Embodiment 3
[0188] The preparation of embodiment 3 formula (A) compound crystal form I
[0189]3.1 Weigh 20mg of the compound of formula (A) into a glass bottle, add acetonitrile (1ml), tetrahydrofuran (1ml), nitromethane (1ml), ethyl acetate (1ml), methyl tert-butyl ether (1ml) respectively , methyl isobutyl ketone (1ml), n-heptane (1ml), diethyl ether (1ml), dichloromethane (1ml), chloroform (1ml), isopropyl acetate (1ml), chloroform / methyl tert-butyl Ether (500μl / 500μl), isopropyl acetate / methyl tert-butyl ether (500μl / 500μl), dichloromethane / toluene (500μl / 500μl), acetonitrile / n-hexane (500μl / 500μl), nitromethane / n-hexane Alkane (500μl / 500μl), ethyl acetate / n-heptane (500μl / 500μl), methyl isobutyl ketone / n-heptane (500μl / 500μl), ethyl acetate / ether (500μl / 500μl), ethyl acetate / petroleum ether (500 μl / 500 μl), dichloromethane / petroleum ether (500 μl / 500 μl), stirred and equilibrated at 25°C for at least 24 hours, filtered, and the solid was dried in air for 10 minutes.
[0190] 3.2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com