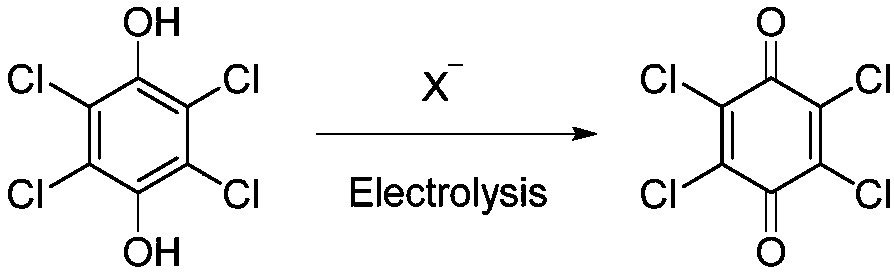
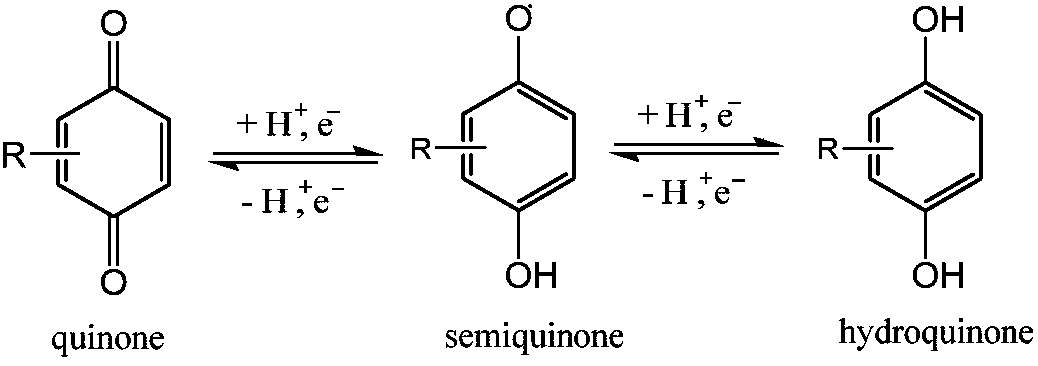
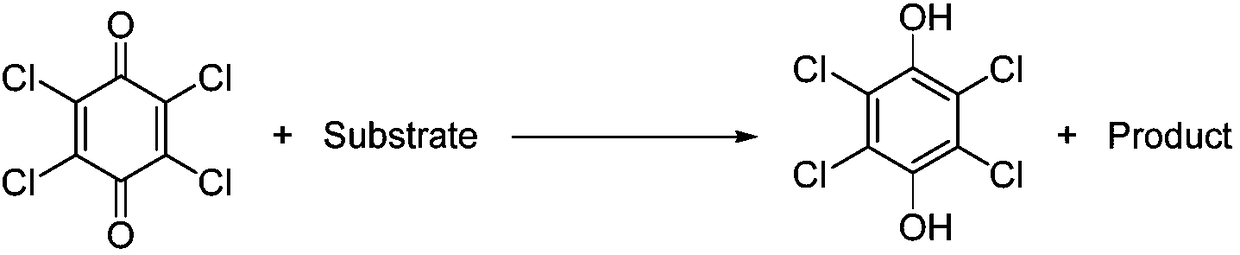
Electrochemical regeneration method of chloranil by taking halogen ions as electric catalyst
A technology of tetrachlorobenzoquinone and electrocatalyst, which is applied in the field of electrochemical regeneration of tetrachlorobenzoquinone, can solve the problems of no domestic and foreign literature reports on regeneration of tetrachlorobenzoquinone, complicated post-treatment, and high equipment requirements, and achieves low cost, The effect of simple and convenient post-processing and simple reaction equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: take the halide ion as the electrochemical regeneration method of the chlorobenzoquinone of electrocatalyst
[0031] In a 50ml single-chamber electrolytic cell, 1240mg (5.0mmol) of tetrachlorohydroquinone and 1030mg (10mmol) of sodium bromide were added to 15ml of methanol solvent, and a small amount of hydrobromic acid was added to make the pH of the reaction system 1-2. With graphite sheet electrode as anode and stainless steel mesh as cathode, at 9mA / cm 2 Electrolyze at a constant current and stir at 25°C. When the energized amount reaches about 4.0 F / mol, the raw materials are basically reacted, and the electrolysis is stopped. The reaction solution is filtered to obtain a yellow solid, which is the product chlorobenzoquinone. Yield: 75%.
Embodiment 2
[0032] Embodiment 2: take the halide ion as the electrochemical regeneration method of the chlorobenzoquinone of electrocatalyst
[0033] In a 50ml single-chamber electrolytic cell, 1240mg (5.0mmol) of tetrachlorohydroquinone and 515mg (5.0mmol) of sodium bromide were added to 15ml of methanol solvent, and a small amount of hydrobromic acid was added to make the pH of the reaction system 1-2. With graphite sheet electrode as anode and stainless steel mesh as cathode, at 9mA / cm 2 Electrolyze at a constant current and stir at 25°C. When the energized amount reaches about 4.0 F / mol, a large amount of raw materials remain, stop the electrolysis, and filter the reaction solution to obtain a yellow solid, which is the product chloranil. Yield: 10%.
Embodiment 3
[0034] Embodiment 3: take the halide ion as the electrochemical regeneration method of the chlorobenzoquinone of electrocatalyst
[0035] In a 50 ml single-chamber electrolytic cell, 1240 mg (5.0 mmol) of tetrachlorohydroquinone and 515 mg (5.0 mmol) of sodium bromide were added to 15 ml of methanol solvent. With graphite sheet electrode as anode and stainless steel mesh as cathode, at 9mA / cm 2 Electrolyze at a constant current and stir at 25°C. When the energized amount reaches about 4.0 F / mol, the raw materials are basically reacted, and the electrolysis is stopped. A dark green solid is attached to the cathode. The reaction solution is filtered to obtain a yellow solid, which is the product Chlorobenzoquinone. Yield: 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com