Method for preparing lithium hydroxide monohydrate by using lithium carbonate
A technology of lithium hydroxide and lithium carbonate, applied in the direction of lithium oxide;/hydroxide, etc., can solve the problems of high energy consumption of evaporation and crystallization, low conversion rate of lithium carbonate, easy conversion into lithium carbonate, etc., so as to improve the conversion rate. , to achieve the effect of no solid and liquid waste discharge, easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
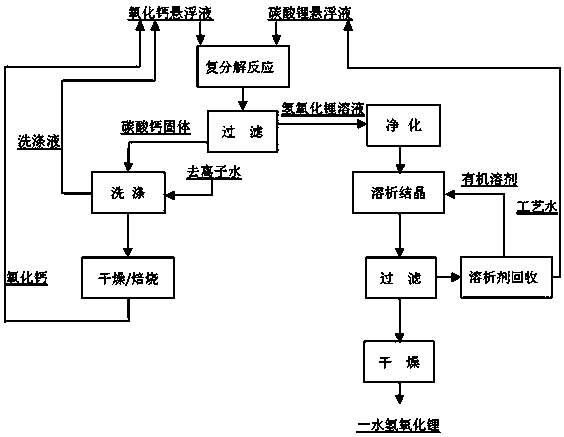
[0027] (1) Metathesis reaction: Weigh 74g of industrial grade lithium carbonate and add 190g of deionized water, weigh 70g of calcium oxide and add 242g of deionized water, preheat to 90°C respectively, and then add them into the reaction kettle for metathesis reaction at 100°C, oxidation The molar ratio of calcium to lithium carbonate is 1:1.25, the mass ratio of calcium oxide and lithium carbonate to deionized water is 1:3, the stirring speed is 400 rpm, the reaction pressure is 101kPa, and the reaction time is 110min.
[0028] (2) Filtration: The slurry after the metathesis reaction is separated from solid and liquid to obtain calcium carbonate solid and lithium hydroxide solution, and the calcium carbonate precipitate is washed with deionized water, and the washing liquid is used in the metathesis reaction to prepare lithium carbonate and calcium oxide The suspension, calcium carbonate solids are dried and calcined, and the resulting calcium oxide is returned to the metathe...
Embodiment 2
[0034] (1) Metathesis reaction: Weigh 74g of industrial grade lithium carbonate and add 190g of deionized water, weigh 61g of calcium oxide and add 215g of deionized water, preheat to 91°C respectively, and then add them into the reaction kettle for metathesis reaction at 108°C, oxidation The molar ratio of calcium to lithium carbonate is 1:1.09, the mass ratio of calcium oxide and lithium carbonate to deionized water is 1:3, the stirring speed is 300 rpm, the reaction pressure is 134kPa, and the reaction time is 100min.
[0035] (2) Filtration: The slurry after the metathesis reaction is separated from solid and liquid to obtain calcium carbonate solid and lithium hydroxide solution, and the calcium carbonate precipitate is washed with deionized water, and the washing liquid is used in the metathesis reaction to prepare lithium carbonate and calcium oxide The suspension, calcium carbonate solids are dried and calcined, and the resulting calcium oxide is returned to the metathe...
Embodiment 3
[0041] (1) Metathesis reaction: Weigh 74g of industrial-grade lithium carbonate and add 190g of deionized water, weigh 56g of calcium oxide and add 200g of deionized water, preheat to 92°C respectively, and then add them into the reaction kettle for metathesis reaction at 120°C, oxidation The molar ratio of calcium to lithium carbonate is 1:1, the mass ratio of calcium oxide and lithium carbonate to deionized water is 1:3, the stirring rate is 200 rpm, the reaction pressure is 200kPa, and the reaction time is 90min.
[0042] (2) Filtration: The slurry after the metathesis reaction is separated from solid and liquid to obtain calcium carbonate solid and lithium hydroxide solution, and the calcium carbonate precipitate is washed with deionized water, and the washing liquid is used in the metathesis reaction to prepare lithium carbonate and calcium oxide The suspension, calcium carbonate solids are dried and calcined, and the resulting calcium oxide is returned to the metathesis rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com