Method for continuously producing diphenyl sulfide in pipelined mode
A diphenyl sulfide and pipeline technology, applied in sulfide preparation, chemical recovery, organic chemistry and other directions, can solve the problems of harsh reaction conditions, cumbersome operation, long reaction time, etc., to reduce the discharge of three wastes, and the reaction effect is good. , construct a simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0043] Embodiment 1-1, a kind of method of pipeline continuous synthesis diphenyl sulfide:
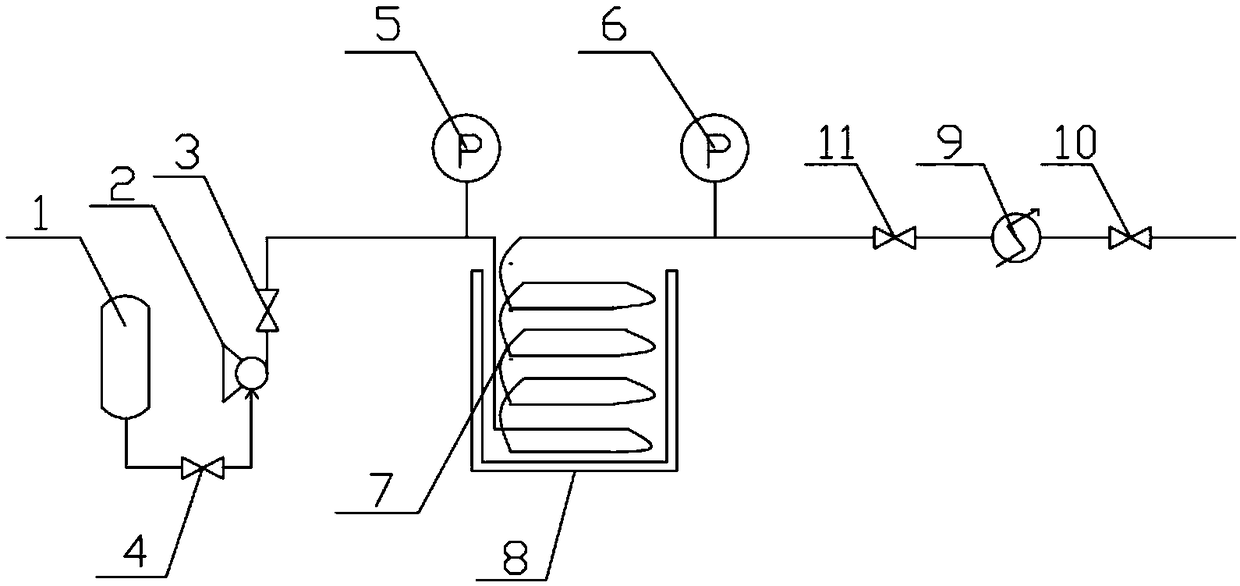
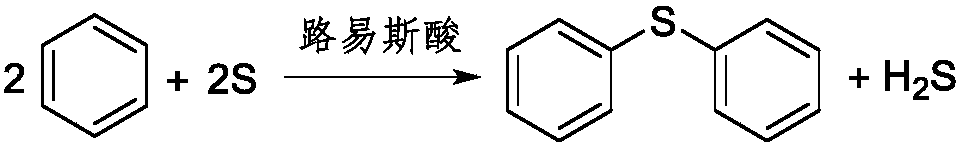
[0044] 780 g of benzene (10 mol), 266.7 g of anhydrous aluminum chloride (2 mol) and 32 g of sulfur (1 mol) were prepared as a reaction liquid, and put into the raw material tank 1. The raw material flowing out of the raw material tank 1 outlet is pumped into the pipelined reactor 7 (the volume of the pipelined reactor 7 is 200ml) by the metering pump 2, and the reaction temperature is 240° C. The reaction pressure is 3MPa, and the flow rate of the metering pump 2 is controlled so that the flow rate of the reaction liquid in the pipeline reactor 7 is about 300ml / h, that is, the reaction time is 40min.
[0045] After the reaction effluent discharged from the outlet of the pipeline reactor 7 is cooled by the cooler 9 (cooled to 35° C.), the gas-liquid phase separation:
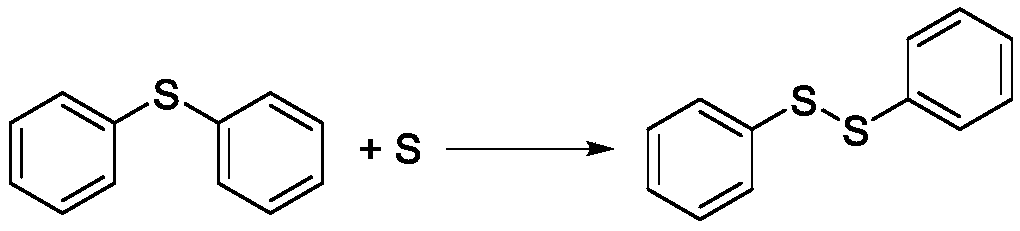
[0046] 1., the gas phase (mainly hydrogen sulfide) of separation gained is passed into 150g, 30wt% sodium hydroxide (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com