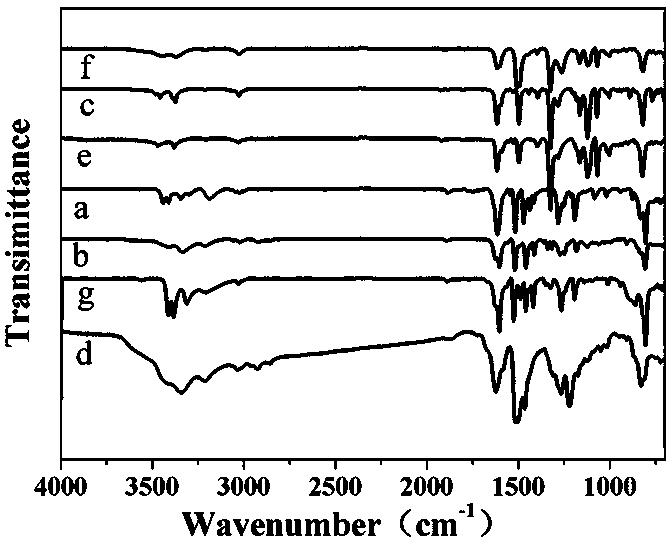
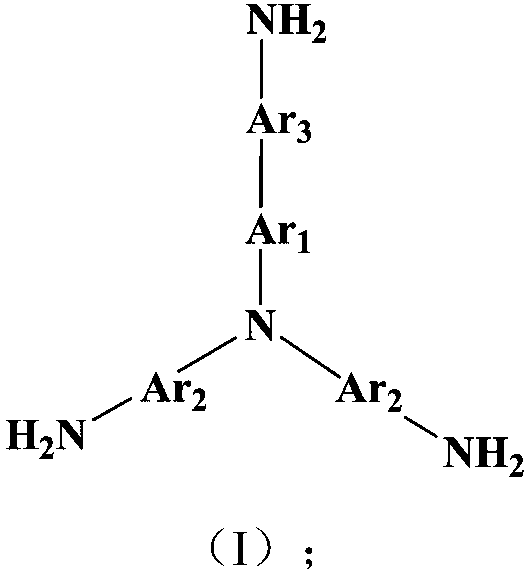
Triamine monomer containing benzothiazole structure and preparation method and application thereof
A technology of benzothiazole and triamine monomers, applied in the direction of organic chemistry, etc., can solve the problems of inability to have both thermal properties and solubility of hyperbranched polyimide, few types of triamine monomers, and unstable thermal properties. , to achieve the effects of improving stability and synthesis rate, low solution and melt viscosity, and mild reaction conditions in the synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] N 1 -(4-aminophenyl)-N 1 -Synthesis of (6-(4-aminophenyl)benzo[d]thiazol-2-yl)benzene-1,4-diamine:
[0056]
[0057] S1. Synthesis of intermediate 6-bromo-N, N-bis(4-nitrophenyl)benzo[d]thiazol-2-amine:
[0058] Add 2.291g (0.01mol) of 2-amino-6-bromobenzothiazole and 7.055mL (0.05mol) of p-fluoronitrobenzene into a 250mL three-necked flask, use DMSO as the solvent, magnetically stir and argon, and heat in an oil bath After reaching 150°C, 3.038g (0.02mol) cesium fluoride (CsF) was added, and the reaction was refluxed for 24h. The reaction solution was poured into ice water, extracted with dichloromethane, decompressed to -101.325kPa to evaporate the solvent, and the product used dichloromethane:n-hexane=1:1 (volume ratio) as the mobile phase and silica gel as the stationary phase. After chromatographic purification, the product was collected and spin-dried, and dried in vacuum at 80° C. for 24 hours to obtain 3.912 g of the product with a yield of 83%. The inter...
Embodiment 2
[0069] N 2 -(5-(4-aminophenyl)benzo[d]thiazol-2-yl)-N 2 -Synthesis of (5-aminothiophen-2-yl)thiophene-2,5-diamine:
[0070]
[0071] S1. Synthesis of intermediate 5-bromo-N, N-bis(5-nitrothiophen-2-yl)benzo[d]thiazol-2-amine:
[0072] Add 2.291g (0.01mol) of 2-amino-5-bromobenzothiazole and 12.752mg (0.05mol) of 2-iodo-5-nitrothiophene into a 250mL three-necked flask with DMSO as the solvent, magnetically stir and argon , heated the oil bath to 150°C, then added 3.038g (0.02mol) cesium fluoride (CsF), and refluxed for 24h. The reaction solution was poured into ice water, extracted with dichloromethane, decompressed to -101.325kPa to evaporate the solvent, and the product used ethyl acetate:petroleum ether=1:10 (volume ratio) as the mobile phase and silica gel as the stationary phase. After chromatographic purification, the product was collected and spin-dried, and dried in vacuum at 80° C. for 24 hours to obtain 3.142 g of the product with a yield of 65%. The intermedia...
Embodiment 3
[0083] N 2 -(6-aminonaphthalen-2-yl)-N 2 -Synthesis of (7-(4-aminophenyl)benzo[d]thiazol-2-yl)naphthalene-2,6-diamine:
[0084]
[0085] S1. Synthesis of intermediate 7-bromo-N, N-bis(6-nitronaphthalen-2-yl)benzo[d]thiazol-2-amine:
[0086] Add 2.291g (0.01mol) of 2-amino-7-bromobenzothiazole and 14.953mg (0.05mol) of 2-iodo-6-nitronaphthalene into a 250mL three-necked flask with DMSO as the solvent, magnetically stir and argon , heated the oil bath to 150°C, then added 3.038g (0.02mol) cesium fluoride (CsF), and refluxed for 24h. The reaction solution was poured into ice water, extracted with dichloromethane, decompressed to -101.325kPa to evaporate the solvent, and the product used dichloromethane:n-hexane=1:1 (volume ratio) as the mobile phase and silica gel as the stationary phase. After chromatographic purification, the product was collected and spin-dried, and dried in vacuum at 80° C. for 24 hours to obtain 3.486 g of the product with a yield of 61%. The intermed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com