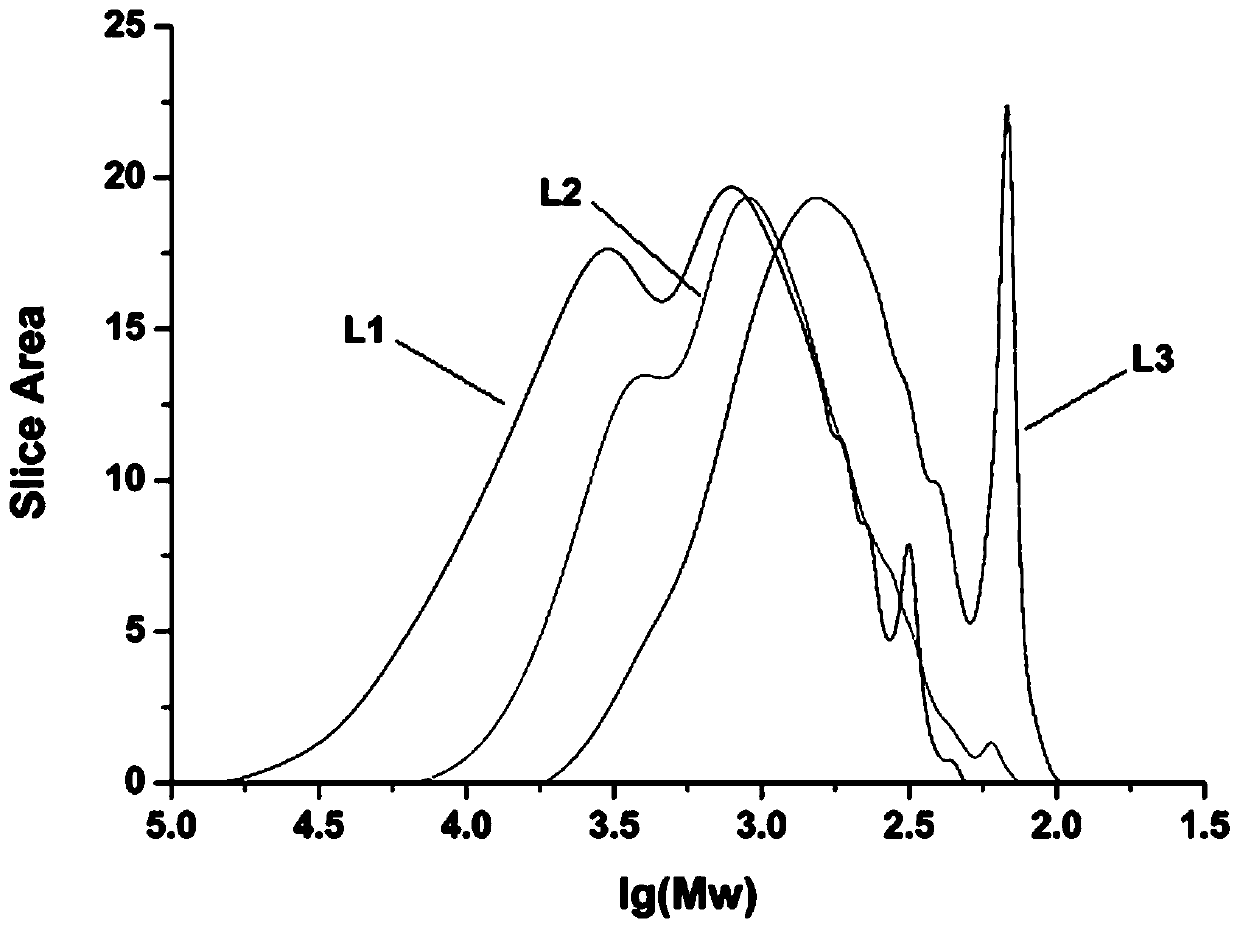
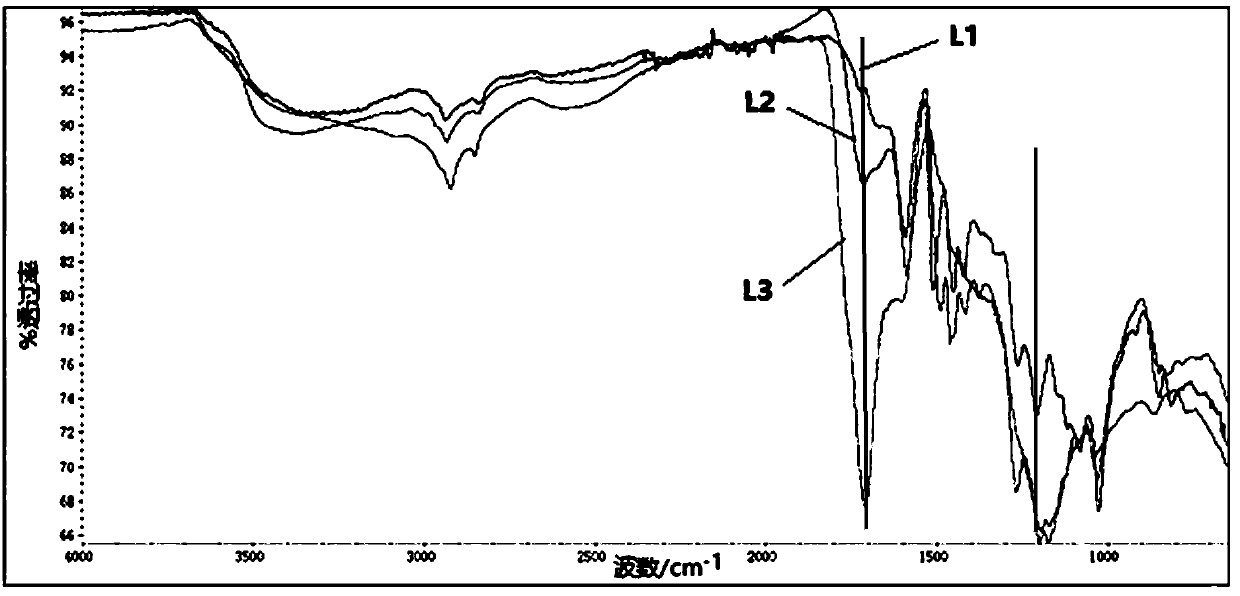
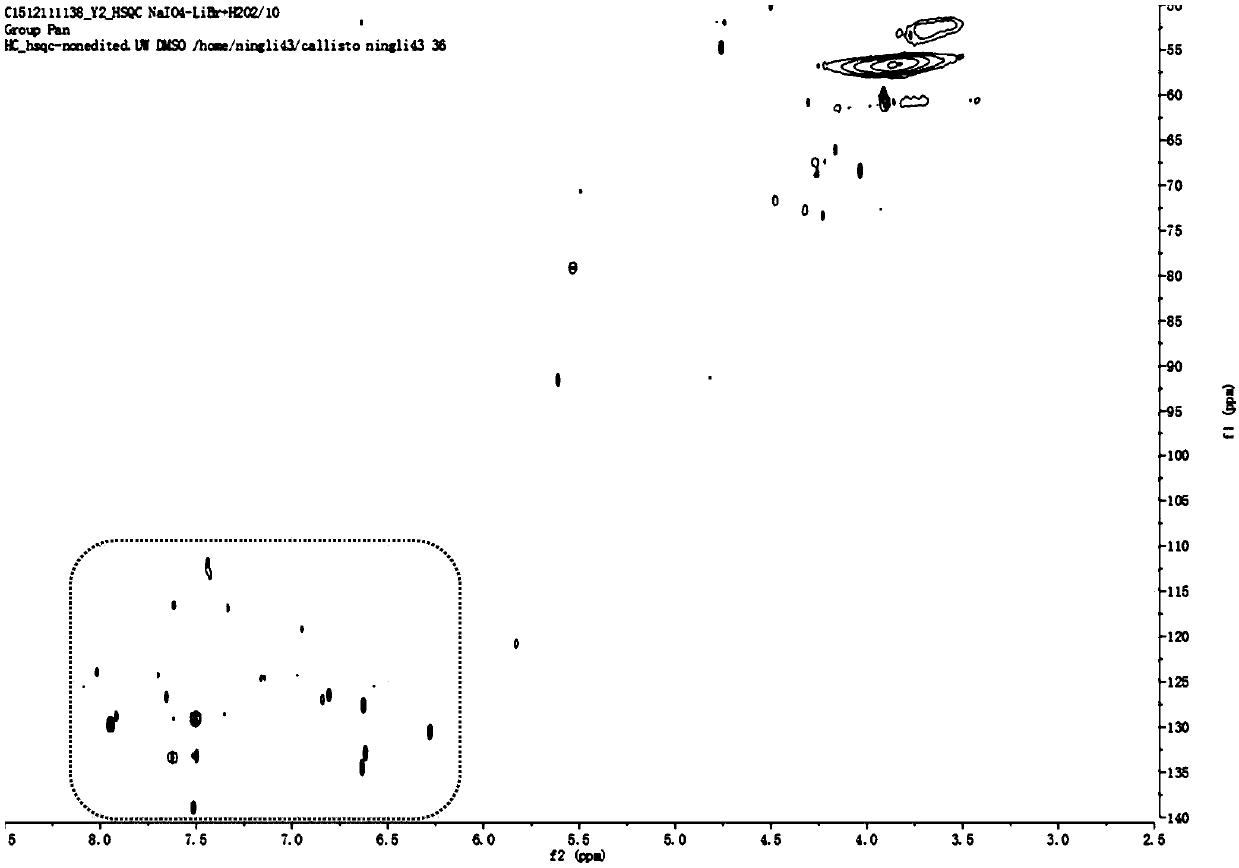
Method for preparing aromatic monomers by virtue of bi-oxidation degradation of lignin
A technology of lignin and double oxidation, applied in the field of lignin conversion, can solve the problems of low replacement rate of lignin, poor material reproducibility, low dispersibility, etc., and achieve the effects of being environmentally friendly, promoting oxidative degradation, and realizing double degradation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Suspend 2 g of Kraft lignin and 0.2 g of TEMPO in 20 mL of ZnCl 2 Add oxygen to the molten salt hydrate, stir evenly, add 50 μL of H 2 SO 4 React at 90 °C for 0.5 h; after the reaction liquid is cooled, filter it with suction and wash it with water for 3 to 4 times; then place it in 20 mL of hydrogen peroxide and react at 20 °C for 5 h; after the reaction liquid is cooled, extract and degrade it with ethyl acetate The product, the organic solvent is removed by rotary evaporation, and the lignin monomer compound can be obtained by vacuum drying, and the yield is 60%.
Embodiment 2
[0025]Suspend 2 g Kraft lignin and 0.3 g TEMPO in 20 mL ZnBr 2 Add oxygen to the molten salt hydrate, stir evenly, add 60 μL of H 2 SO 4 React at 90 °C for 0.5 h; after the reaction liquid is cooled, filter it with suction and wash it with water for 3 to 4 times; then place it in 20 mL of hydrogen peroxide and react at 20 °C for 5 h; after the reaction liquid is cooled, extract and degrade it with ethyl acetate The product, the organic solvent was removed by rotary evaporation, and the lignin monomer compound was obtained by vacuum drying, and the yield was 62%.
Embodiment 3
[0027] Put 2 g organic solvent lignin and 0.5 g sodium tungstophosphate 2Na 2 O•12WO 3 •P 2 o 5 •18H 2 O suspended in 20 mL CuBr 2 Add oxygen to the molten salt hydrate, stir evenly, add 100 μL of hydrochloric acid and react at 100 °C for 1 h; after the reaction solution is cooled, filter it with suction and wash it with water for 3 to 4 times; then place it in 20 mL of hydrogen peroxide , reacted at 30 ℃ for 8 h, after the reaction solution was cooled, the degradation product was extracted with ethyl acetate, the organic solvent was removed by rotary evaporation, and the lignin monomer compound was obtained by vacuum drying with a yield of 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com