Method for simultaneously determining contents of two active ingredients in Salvia miltiorrhiza-pseudo-ginseng preparation
A preparation, the technology of notoginseng, applied in the field of medicine, can solve the problems of effective quality control, time-consuming, and inability to realize prescriptions, and achieve the effect of fast method and improved work efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
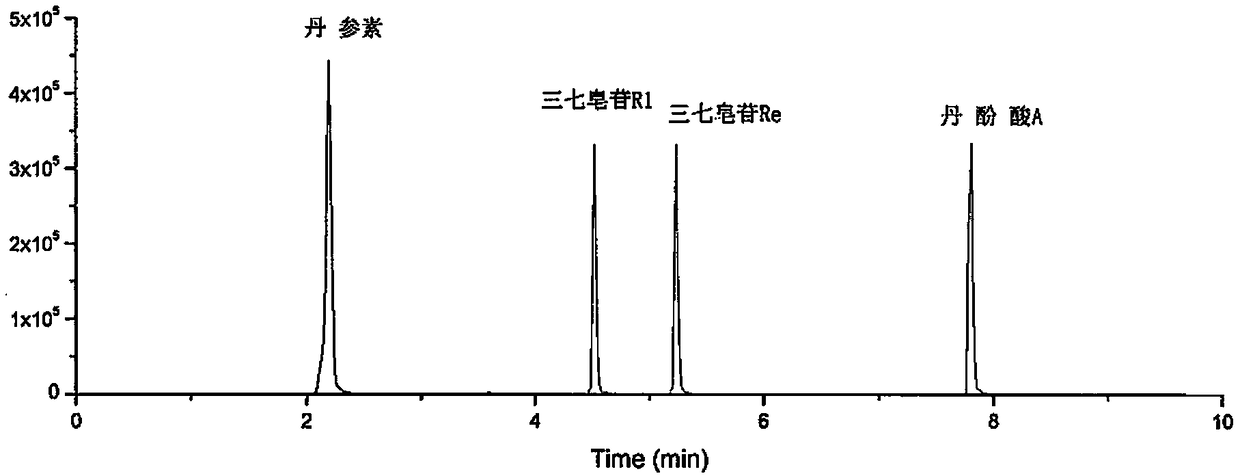
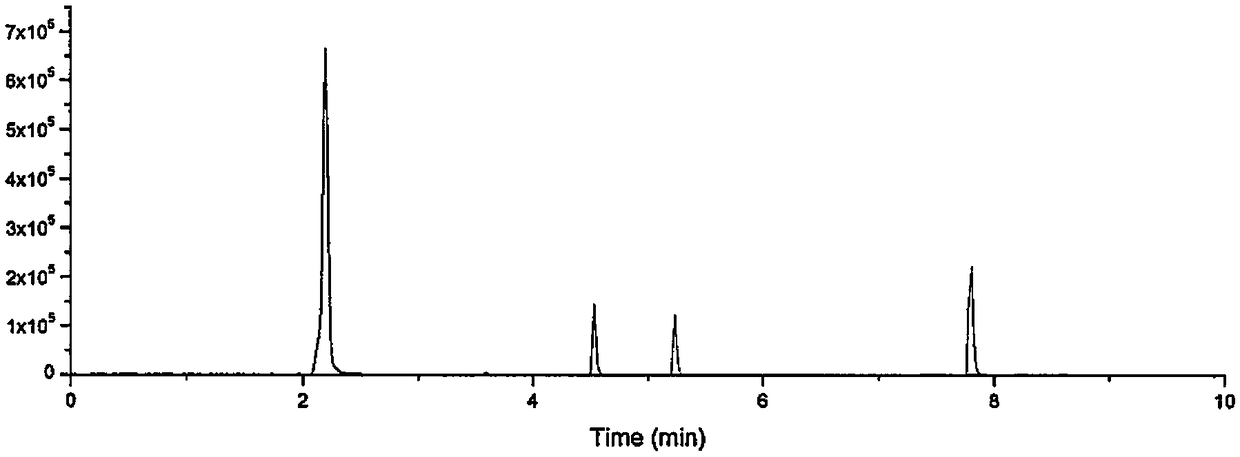
[0023] Example 1: Detection of the content of two types of four active ingredients in Danqi Soft Capsules
[0024] (1) Instrument
[0025] Ultra-high performance liquid chromatography (UPLC, Waters, USA); series API 5500 triple quadrupole mass spectrometer (Applied Biosystems, USA), equipped with Waters UPLC autosampler, column thermostat, and data acquisition was completed by Analyst software.
[0026] (2) Instrument operating conditions
[0027] Liquid chromatography conditions: Waters ACQUITY UPLC BEH C18 column (2.1mm×50mm, 1.7μm); injection volume is 1μL; flow rate is 0.4mL / min; column temperature is 30°C; mobile phase A has a volume ratio of 0.1: 99.9 mixed solution of formic acid and water, mobile phase B is a mixed solution of formic acid and acetonitrile with a volume ratio of 0.1:99.9; carry out gradient elution, the gradient conditions are:
[0028]
[0029] Mass spectrometry conditions: ion source is Turbo V; ionization mode is ESI - The air curtain gas volum...
Embodiment 2
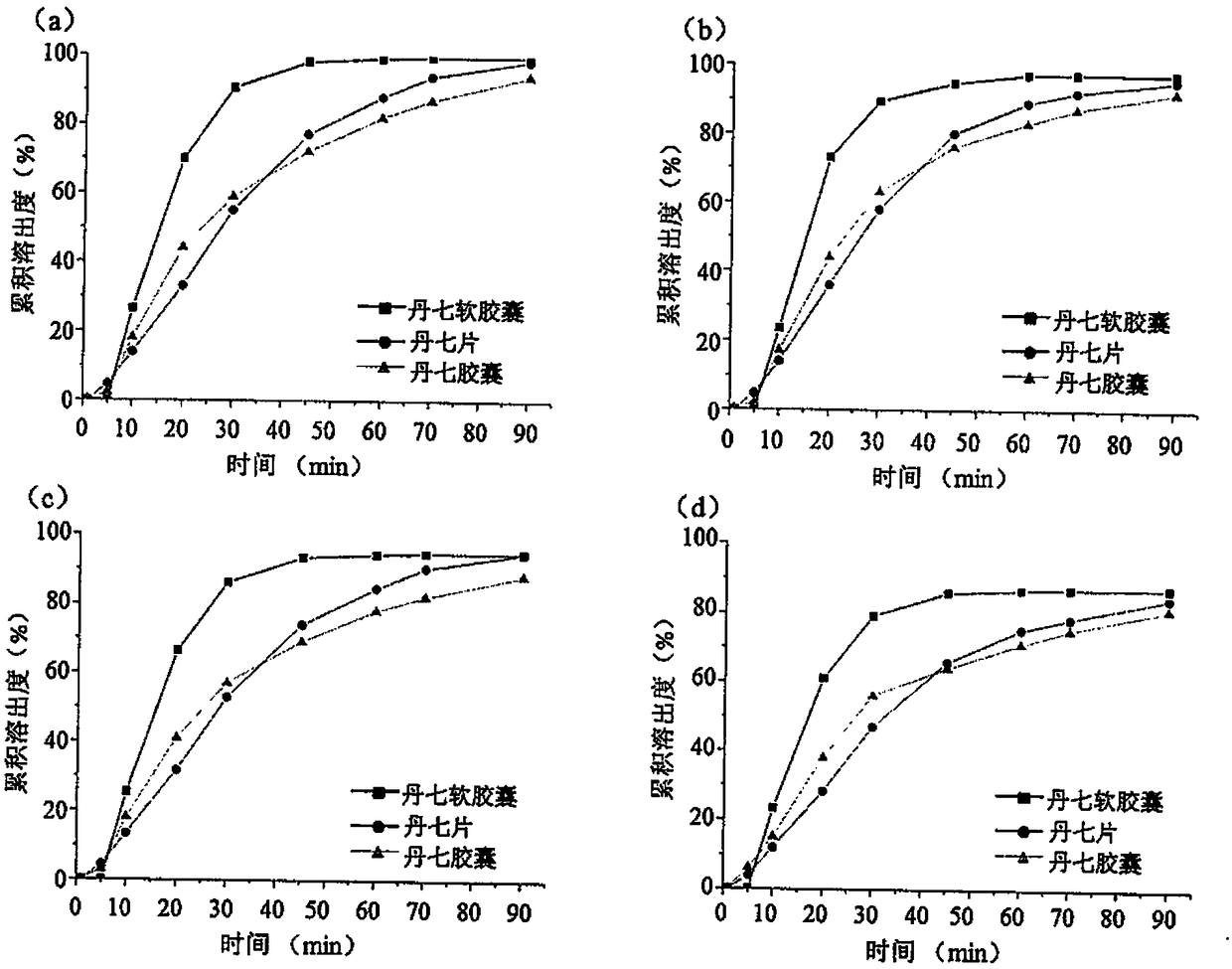
[0045] Example 2 Determination of Dissolution Rates of Two Kinds of Four Active Components of Danqi Soft Capsules
[0046] (1) Conditions and determination of dissolution rate
[0047] Measure 900ml of degassed dissolution medium (1% SDS aqueous solution), heat the medium temperature up to (37±0.5)°C, take 6 Danqi soft capsules, 6 Danqi tablets and 6 Danqi tablets Seven capsules, 1 capsule in each basket, 6 repetitions of each preparation, test at 100r / min, 2mL samples were taken at fixed points at 1, 5, 10, 20, 30, 45, 60, 70, 90min, At the same time, add the same medium at the same temperature and the same amount, add methanol to the sample to 4mL, shake well to pass through a 0.22μm PTFE filter membrane, take the subsequent filtrate, inject 1μL by UPLC-TQ MS method, measure the content of the target compound, and calculate the target compound cumulative dissolution rate. Taking time as the abscissa and the cumulative dissolution rate as the ordinate, the dissolution curve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com