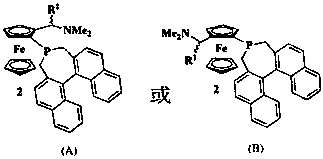Chiral ferrocene diphosphine ligands, preparation method and application thereof
A technology for chiral ferrocene and bisphosphine ligands is applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxy compounds, etc. The effect of stable moisture and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
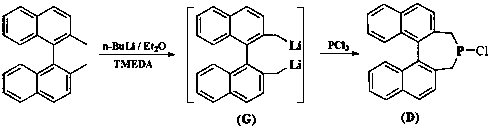
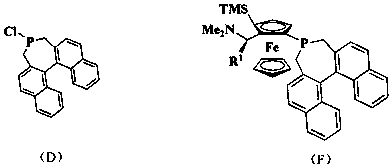
[0039] Add dropwise ( S )-8 (21 g, 74.5 mmol) in ether, after the dropwise addition, slowly add the dried TMEDA (28.6 mL, 192 mmol), after the addition, the reaction solution was raised to room temperature for 24 h. Stand still, extract the supernatant with a syringe to obtain a large amount of deep purple solid, wash twice with dry ether, extract the supernatant in the same way, and remove the remaining solvent with an oil pump to obtain 13.1 g of dilithium salt, which can be obtained without purification. used directly in the next reaction. Weigh the lithium salt (2.94 g, 10 mmol) obtained in the previous step in the glove box and add it to a one-necked bottle, inject 60 mL of dry n-hexane under a nitrogen atmosphere, and then slowly inject phosphorus trichloride (0.87 mL, 10 mmol), at room temperature Stir for 12 h. The insoluble matter was removed by suction filtration, and the solvent was evaporated to dryness to obtain a brown solid crude product, which was recrystalli...
Embodiment 2
[0043]
[0044] by( R )-8 is that raw material obtains phosphine chloride ( R )-5. Phosphine chloride ( R )-5 is that the raw material obtains the orange-yellow bubbly product ( R , S , R )-2 3.74 g, yield 66% (ethyl acetate: petroleum ether: triethylamine=1:5:0.5%). 1 H NMR (400Hz, CDCl 3 ) δ 7.97-7.81 (m, 3H), 7.69 (t, J = 8.4 Hz, 2H),7.45-7.37 (m, 2H), 7.33-7.28 (m, 1H), 7.28-7.12 (m,3H), 6.74 ( d, J = 8.3 Hz, 1H), 4.39-4.22 (m, 1H), 4.11 (dd, J = 6.9, 2.7 Hz, 1H), 4.07-4.04 (m, 1H), 4.02 (s, 5H), 3.43 -3.30 (m, 1H), 2.98- 2.79 (m, 1H), 2.80-2.66 (m, 2H), 2.54(t, J = 13.0 Hz, 1H), 2.14 (s, 6H), 1.25 (d, J = 6.8 Hz, 3H); 13 C NMR (101 Hz, CDCl 3 ) δ 135.48, 135.17, 133.83, 132.70, 132.33, 132.23, 132.07, 131.83,128.93, 128.20, 128.13, 127.57, 126.95, 126.72, 126.54, 125.79, 125.63,124.84, 124.46, 96.83, 73.63, 70.30, 69.47, 69.26, 67.48, 56.79, 39.17, 32.80, 29.50, 7.89; 31 P NMR (162 Hz, CDCl 3 ) δ -6.58; HRMS (ESI) calcd for C 36 h 35 FeNP[M+H] + : ...
Embodiment 3
[0046]
[0047] To the monophosphine intermediate ( R , S , R )-2 (284 mg, 0.5 mmol) in glacial acetic acid solution was added bis(3,5-dimethylphenyl) phosphine 3a (133 mg, 0.55 mmol), the reaction temperature rose to 82 ~ 107 ° C, nuclear magnetic monitoring to the reaction End. Cooled to room temperature, dichloromethane diluted the reaction solution, washed with water, saturated sodium bicarbonate solution and saturated brine successively, dried, spin-dried, and column chromatography (ethyl acetate:petroleum ether=1:30) gave orange bisphosphine Ligand( R , S , R )-Ia275 mg, yield 72%. 1 H NMR (400Hz, CDCl 3 ) δ 8.03 (d, J = 8.3 Hz, 1H), 7.96 (d, J = 8.2 Hz, 1H), 7.85 (dd, J = 16.0, 8.2 Hz, 2H), 7.77 (d, J = 8.3 Hz, 1H), 7.54-7.40 (m, 2H), 7.37 (t, J= 7.5 Hz, 1H), 7.24-7.14 (m, 3H), 7.11 (d, J = 8.5 Hz, 1H), 6.96 (d, J = 7.1Hz , 3H), 6.89-6.75 (m, 3H), 4.27 (s, 5H), 4.14 (s, 1H), 4.11 (s, 1H), 3.81(t, J = 5.7 Hz, 1H), 3.75 (s, 1H), 3.25 (d, J = 14.4 Hz, 1H), 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com