Preparation method of organic silicon polymer with anthracene-protected alpha-cyanoacryloyloxy structure
A technology of cyanoacryloyloxy and organosilicon compounds, which is applied in the field of preparation of organosilicon compounds, can solve the problems of harsh reaction conditions, many by-products, and low energy consumption, and achieve easy separation and purification, mild reaction conditions, and product make up a single effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
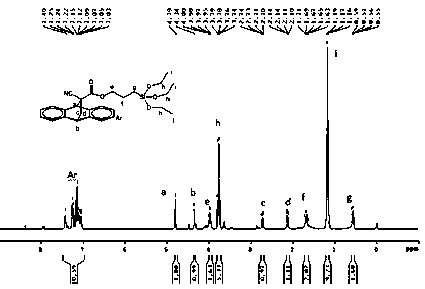
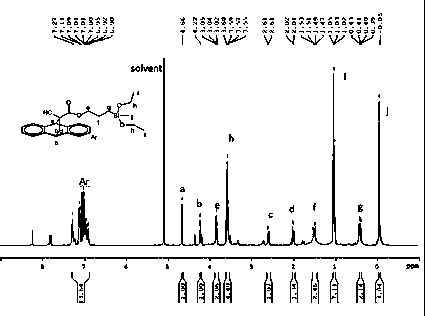
[0042] Add 20mL of DMF solvent to a 100mL three-necked flask equipped with a condenser, a thermometer and electromagnetic stirring, and add 3.13g (0.01mol) anthracene-protected α-cyanoacrylate potassium salt, 2.48g (0.0103mol) γ-chloro Propyltriethoxysilane, 0.311g halogenation reaction catalyst potassium iodide, 0.0319g esterification reaction catalyst 4-dimethylaminopyridine, then heat up to 120°C, react at this temperature for 2h and then cool down to room temperature, and filter the crude reaction product After removing the by-product KCl, slowly add the collected filtrate dropwise at a rate of 1-2 drops per second to 100mL of 12% sodium chloride aqueous solution under stirring, and let stand for 2h after the dropwise addition, and wait for the product After settling, separate the liquid to remove the upper water layer, add 40mL of dichloromethane lysates to the collected organic layer, then add 80mL of deionized water in the dichloromethane solution, wash off the residual ...
Embodiment 2
[0044] Add 50mL of DMAC (N,N-dimethylacetamide) solvent to a 250mL three-necked flask equipped with a condenser, a thermometer and electromagnetic stirring, and add 3.13g (0.01mol) of anthracene-protected potassium α-cyanoacrylate under stirring at room temperature Salt, 11.91g (0.0495mol) γ-chloropropyl triethoxysilane, 0.783g halogenation reaction catalyst potassium bromide, 0.1565g esterification reaction catalyst 2-dimethylaminopyridine, then be heated to 120 ℃, at this Cool down to room temperature after reacting for 5 hours at high temperature. After filtering the crude reaction product to remove the by-product KCl, slowly add the collected filtrate dropwise to 180 mL of sodium chloride with a mass fraction of 13% under stirring at a rate of 1-2 drops per second. In the aqueous solution, let it stand for 2 hours after the dropwise addition. After the product settles, separate the liquid to remove the upper water layer, add 110 mL of dichloromethane dissolved product to th...
Embodiment 3
[0046] Add 20mL DMSO (dimethyl sulfoxide) solvent to a 100mL three-necked flask equipped with a condenser tube, a thermometer and electromagnetic stirring, and add 3.13g (0.01mol) anthracene-protected α-cyanoacrylate potassium salt under stirring at room temperature, 2.98g ( 0.015mol) γ-chloropropyltrimethoxysilane, 0.157g halogenation reaction catalyst sodium iodide, 0.017g esterification reaction catalyst 4-aminopyridine, then heat up to 100°C, react at this temperature for 5h and then cool down to room temperature After the reaction crude product was filtered to remove the by-product KCl, the collected filtrate was slowly added dropwise at a rate of 1-2 drops per second to 70mL of 9% sodium chloride aqueous solution with a mass fraction of 9% under stirring. Set aside for 6h, after the product settles, remove the upper water layer by separating, add 20mL of chloroform to dissolve the product in the collected organic layer, and then add 90mL of deionized water to the chlorofo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com