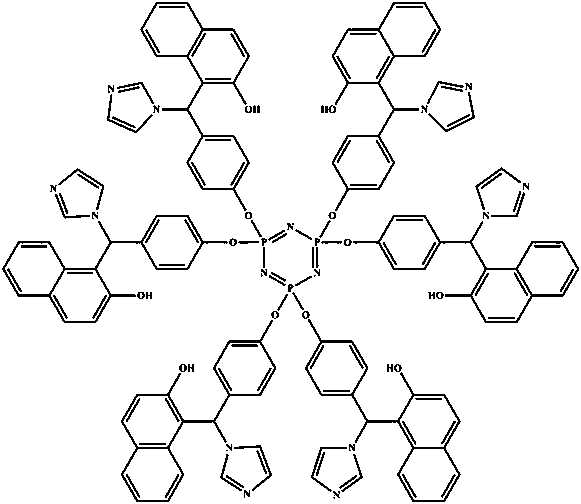
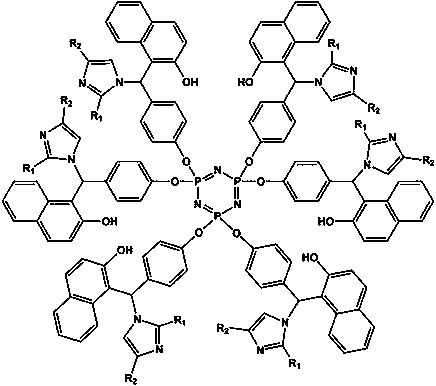
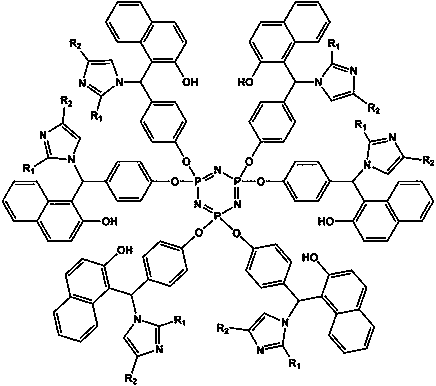
Epoxy resin curing accelerator based on cyclotriphosphazene and imidazole compound as well as preparation method and application method thereof
A technology of epoxy resin curing and imidazole compounds, which is applied in the field of epoxy electronic packaging, can solve the problems of long-term storage of single-component systems, shorten the service life of materials, and reduce the curing temperature, etc., so as to improve latent performance and room temperature storage stability The effect of raising and lowering activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Weigh 14.76 g p-hydroxybenzaldehyde and 20 mL triethylamine, dissolve in 40 mL tetrahydrofuran, 66 o Under C reflux and stirring, the tetrahydrofuran solution of hexachlorocyclotriphosphazene (6.95 g of hexachlorocyclotriphosphazene dissolved in 40 mL of tetrahydrofuran) was added dropwise to it, and the addition was completed within 40 minutes. After the reaction was continued for 8 h, the turbid liquid was suction filtered to obtain a light yellow filtrate. After the solvent was removed by rotary evaporation, washed twice with ethanol, filtered to obtain a white powder, and finally recrystallized in ethyl acetate twice, the precipitate was dried in a vacuum drying oven for 12 h to obtain a pale yellow powder, which is Aldehyde phenoxy) cyclotriphosphazene.
[0025] Weigh 4.31 g of hexa(p-aldehyde phenoxy) cyclotriphosphazene prepared above, 4.33 g 2-naphthol and 2.04 g imidazole, dissolve them in 60 mL isopropanol, adjust the pH of the reaction environment with hydrochlo...
Embodiment 2
[0028] Weigh 14.76 g p-hydroxybenzaldehyde and 20 mL triethylamine, dissolve in 40 mL tetrahydrofuran, 66 o Under C reflux and stirring, the tetrahydrofuran solution of hexachlorocyclotriphosphazene (6.95 g of hexachlorocyclotriphosphazene dissolved in 40 mL of tetrahydrofuran) was added dropwise to it, and the addition was completed within 40 minutes. After the reaction was continued for 8 h, the turbid liquid was suction filtered to obtain a light yellow filtrate. After the solvent was removed by rotary evaporation, washed twice with ethanol, filtered to obtain a white powder, and finally recrystallized in ethyl acetate twice, the precipitate was dried in a vacuum drying oven for 12 h to obtain a pale yellow powder, which is Aldehyde phenoxy) cyclotriphosphazene.
[0029] Weigh 4.31 g of hexa(p-aldehyde phenoxy) cyclotriphosphazene prepared above, 4.33 g 2-naphthol and 2.04 g imidazole, dissolve them in 60 mL isopropanol, adjust the pH of the reaction environment with hydrochlo...
Embodiment 3
[0032] Weigh 14.76 g p-hydroxybenzaldehyde and 20 mL triethylamine, dissolve in 40 mL tetrahydrofuran, 66 o Under C reflux and stirring, the tetrahydrofuran solution of hexachlorocyclotriphosphazene (6.95 g of hexachlorocyclotriphosphazene dissolved in 40 mL of tetrahydrofuran) was added dropwise to it, and the addition was completed within 40 minutes. After the reaction was continued for 8 h, the turbid liquid was suction filtered to obtain a light yellow filtrate. After the solvent was removed by rotary evaporation, washed twice with ethanol, filtered to obtain a white powder, and finally recrystallized in ethyl acetate twice, the precipitate was dried in a vacuum drying oven for 12 h to obtain a pale yellow powder, which is Aldehyde phenoxy) cyclotriphosphazene.
[0033] Weigh 4.31 g of hexa(p-aldehyde phenoxy) cyclotriphosphazene prepared above, 4.33 g 2-naphthol and 2.04 g imidazole, dissolve them in 60 mL isopropanol, adjust the pH of the reaction environment with hydrochlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| epoxy equivalent | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com