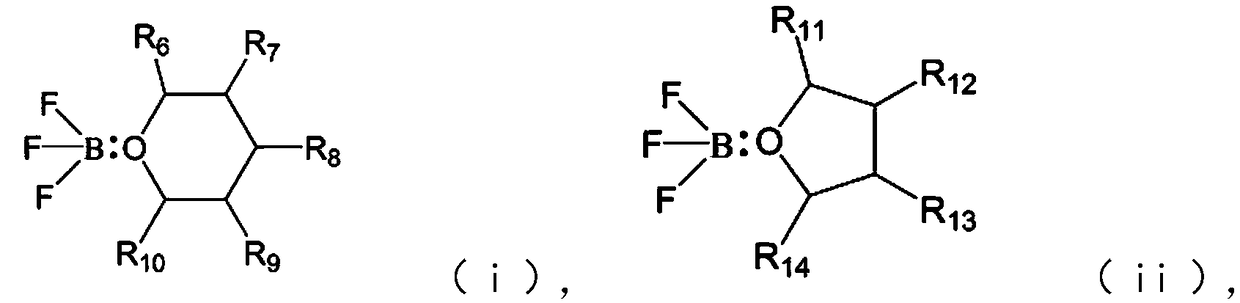
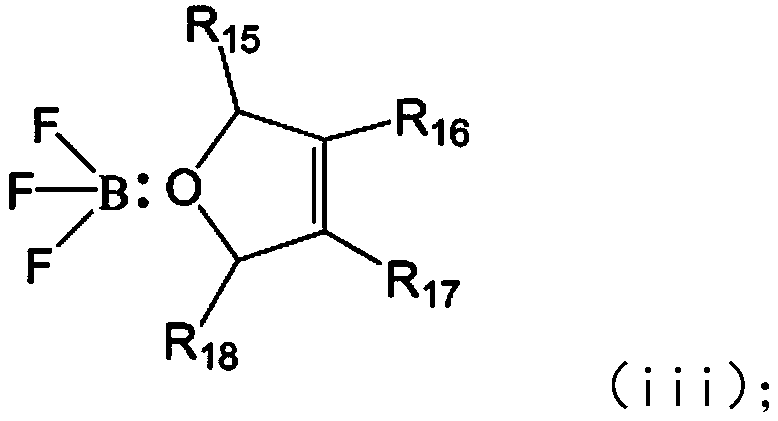
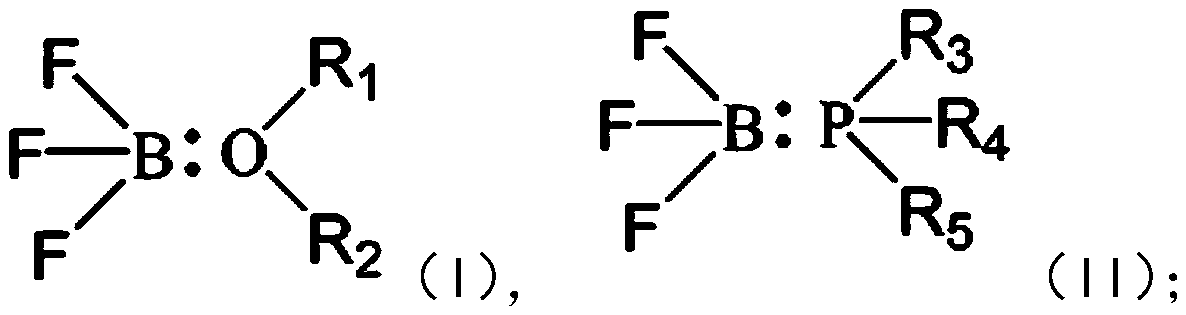Electrolyte additive, electrolyte containing same, lithium ion battery and apparatus
An electrolyte additive and electrolyte technology, applied in secondary batteries, secondary battery repair/maintenance, circuits, etc., can solve the problem of restricting the development of high-voltage lithium-ion batteries, poor cycle performance, and reduced charging and discharging efficiency of lithium-ion batteries To achieve the effect of optimizing the surface film of positive and negative electrodes, improving cycle performance, and inhibiting further contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0103] The preparation method of this electrolyte can refer to as follows:
[0104] (a) removing impurities and water after purifying the organic solvent;
[0105] In this step, it is preferred to mix the cyclic carbonate solvent and the linear carbonate solvent to obtain a mixed organic solvent, and then remove impurities and water from the mixed organic solvent;
[0106] (b) adding the lithium salt to the organic solvent after the impurity removal in step (a), to obtain the basic electrolyte;
[0107] (c) adding an electrolyte additive having a mass of 0.5 to 3% of the basic electrolyte to the basic electrolyte obtained in step (b), to obtain the electrolyte;
[0108] At the same time, in step (b), by adjusting the amount of lithium salt added, the concentration of lithium salt in the obtained electrolyte is controlled at about 1.0 mol / L.
[0109] The electrolyte solution prepared by the above method can be further used to prepare a corresponding lithium secondary battery....
Embodiment 1
[0117](1) cyclic carbonate solvent ethylene carbonate (EC) and linear carbonate solvent ethyl methyl carbonate (EMC) and diethyl carbonate (DEC) by mass ratio EC:EMC:DEC=3:5: 2 Mix, and use molecular sieves, calcium hydride, lithium hydride to purify and remove impurities and water;
[0118] (2) At room temperature, the conductive lithium salt LiPF 6 Dissolve in the solvent obtained in step (1), the final concentration of the conductive lithium salt is 1.0mol / L, and stir evenly to obtain the basic electrolyte;
[0119] (3) Boron trifluoride tetrahydrofuran complex is added to the basic electrolyte prepared in step (2) in an amount of 1% of the mass of the basic electrolyte to obtain the final electrolyte.
Embodiment 2-3、 comparative example 1
[0121] Except the following table 1 parameter, other parameters and preparation method of embodiment 2-3 and comparative example 1 are the same
[0122] Example 1.
[0123] Table 1 embodiment 2-3, comparative example 1 electrolyte composition parameter
[0124]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com