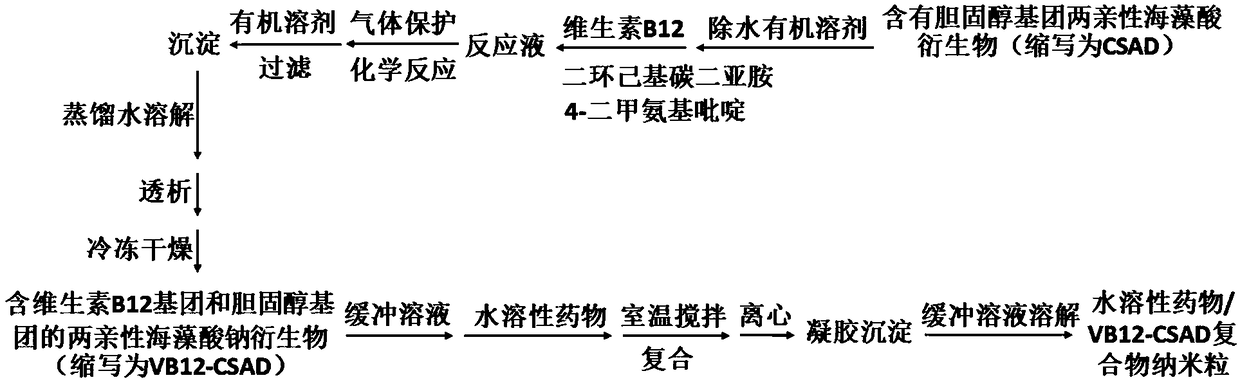
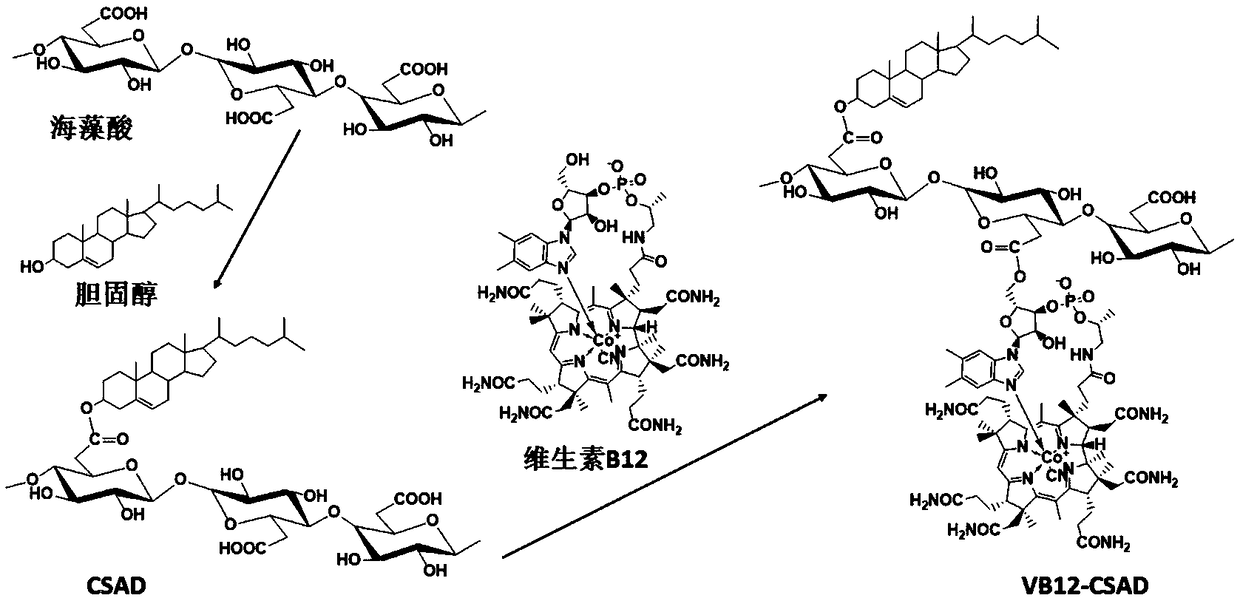
Vitamin B12 group containing amphiphilic sodium alginate derivative and preparation method and application thereof
A sodium alginate and B12 technology, which is applied in the field of amphiphilic sodium alginate derivatives and its preparation, can solve the problems that have not yet been reported on sodium alginate oral nanoparticles, achieve the enhancement of small intestine targeted absorption performance, and maintain biological activity , The effect of low synthesis reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] (1) According to the ratio of CSAD (the substitution degree of cholesterol group is 15%): dehydrated ethyl acetate = 0.5g: 10mL, add dehydrated ethyl acetate to CSAD, and stir at room temperature at 100 rpm under nitrogen protection Dissolve in 24 hours to obtain CSAD solution;
[0057] (2) According to the ratio of vitamin B12 (VB12): dehydrated ethyl acetate = 0.05g: 3mL, add dehydrated ethyl acetate to VB12 to dissolve;
[0058] (3) According to the ratio of dicyclohexylcarbodiimide: 4-dimethylaminopyridine: dehydrated ethyl acetate = 0.1g: 0.05g: 3mL, dicyclohexylcarbodiimide and 4-dimethylaminopyridine Add dehydrated ethyl acetate to dissolve;
[0059] (4) Slowly add the solution obtained in step 2 and step 3 to the CSAD solution in step 1, and react for 20 hours at 500 rpm under nitrogen protection;
[0060] (5) Precipitate the reaction solution in step 4 with isopropanol, filter, and collect the precipitate;
[0061] (6) Add 10 mL of distilled water to the pre...
Embodiment 2
[0072] (1) According to the ratio of CSAD (the degree of substitution of cholesterol groups is 3%): dehydrated dimethylformamide = 1g: 0.5mL, add dehydrated dimethylformamide to CSAD, under the protection of helium at 500 rev / min, stirred at room temperature for 18 hours and dissolved to obtain a CSAD solution;
[0073] (2) According to the ratio of VB12: dehydrated dimethylformamide = 0.5g: 5mL, add dehydrated dimethylformamide to VB12 to dissolve;
[0074](3) According to the ratio of dicyclohexylcarbodiimide: 4-dimethylaminopyridine: dehydrated dimethylformamide = 0.3g: 0.3g: 1mL, dicyclohexylcarbodiimide and 4-dimethylformamide Add dehydrated dimethylformamide to aminopyridine to dissolve;
[0075] (4) Slowly add the solution obtained in step 2 and step 3 to the CSAD solution in step 1, and react for 2 hours at 100 rpm under the protection of helium;
[0076] (5) Precipitate the reaction solution in step 4 with ethanol, filter, and collect the precipitate;
[0077] (6) ...
Embodiment 3
[0083] (1) According to the ratio of CSAD (the degree of substitution of cholesterol groups is 8%): dehydrated dimethyl sulfoxide = 0.6g: 35mL, add dehydrated dimethyl sulfoxide to CSAD, under the protection of argon at 300 Rotation / min Stir at room temperature for 1 hour to dissolve, and obtain CSAD solution;
[0084] (2) According to the ratio of VB12: dehydrated dimethyl sulfoxide = 0.02g: 1mL, add dehydrated dimethyl sulfoxide to VB12 to dissolve;
[0085] (3) According to the ratio of dicyclohexylcarbodiimide: 4-dimethylaminopyridine: dehydrated dimethyl sulfoxide = 0.5g: 0.1g: 5mL, dicyclohexylcarbodiimide and 4-dimethyl Add dehydrated dimethyl sulfoxide to aminopyridine to dissolve;
[0086] (4) Slowly add the solution obtained in step 2 and step 3 to the CSAD solution in step 1, and react for 48 hours at 400 rpm under the protection of argon;
[0087] (5) Precipitate the reaction solution in step 4 with acetone, filter, and collect the precipitate;
[0088] (6) Add ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com