Magnetic microporous lithium adsorbent and its preparation method and application
A lithium adsorption and microporous technology, which is applied in chemical instruments and methods, lithium compounds, and other chemical processes, can solve the problems of insignificant advantages in the adsorption capacity of adsorbents, insignificant advantages in specific surface area of adsorbents, poor fluidity and permeability, etc. Problems, to achieve significant static adsorption capacity and dynamic working adsorption capacity, which is conducive to concentration and enrichment and subsequent utilization processing, and the effect of long cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
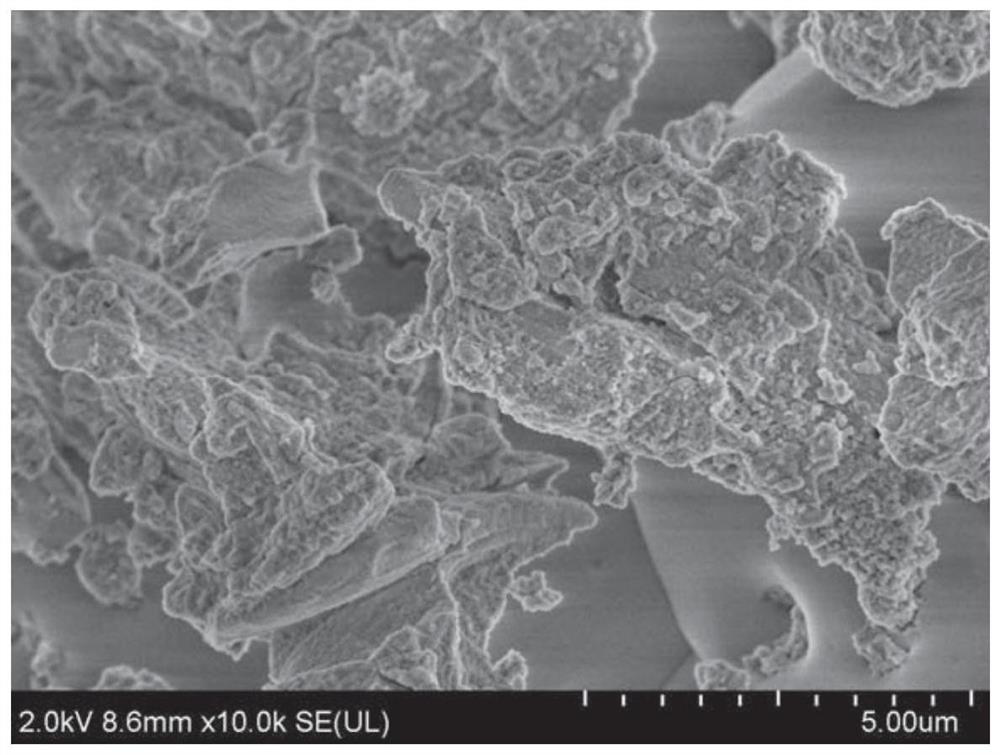
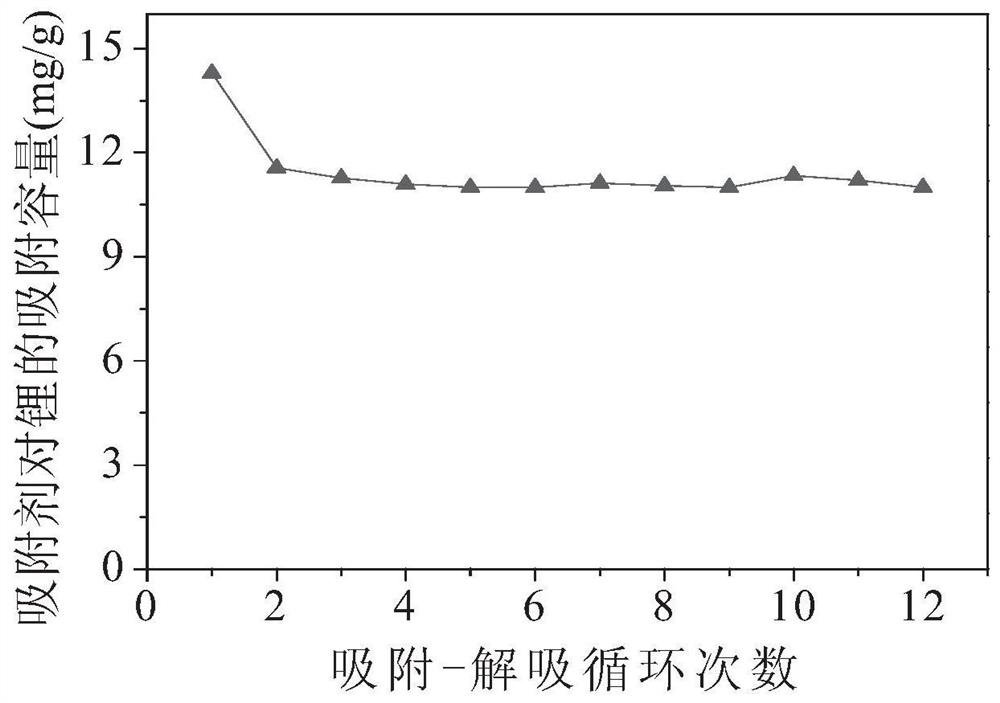
Image
Examples
preparation example Construction
[0025] As an aspect of the technical solution of the present invention, it relates to a preparation method of a magnetic microporous lithium adsorbent, comprising:
[0026] Using a surface modifier to modify the surface of the magnetic material to obtain a surface-modified magnetic material;
[0027] The soluble lithium salt, soluble aluminum salt and alkali are added to the alcohol dispersion of the surface-modified magnetic material for reaction, thereby preparing the magnetic microporous lithium adsorbent.
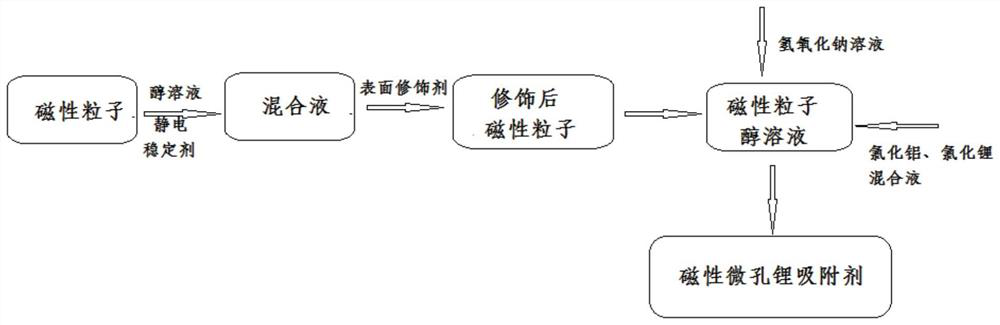
[0028] see figure 1 , is a schematic diagram of the synthesis process of the magnetic microporous lithium adsorbent, and in some embodiments, specifically includes:
[0029] The magnetic material is evenly dispersed in the alcohol solution containing the electrostatic stabilizer, and the surface modification agent is added for reaction, so as to obtain the magnetic material after the surface modification.
[0030] Further, the reaction temperature is 20-50°C.
[0031...
Embodiment 1
[0070] 5.0 g of magnetic Fe prepared by co-precipitation method 3 o 4 The particles were evenly dispersed into 80mL of ethanol solution containing 0.5% sodium acetate, and at 20°C, slowly added 0.58mL of 3-aminopropyltriethoxysilane and refluxed for 12h to obtain the surface-modified magnetic Fe 3 o 4 particle. Magnetic Fe 3 o 4 The particles were washed three times alternately with ethanol and deionized water. It was dispersed into 60 mL of ethanol solution containing sodium acetate. Lithium chloride and aluminum chloride solution were mixed uniformly according to the ratio of the amount of substances 0.6:1 and ultrasonically dispersed, then added dropwise at the speed of 200mL / h to the magnetic Fe 3 o 4 Particles in sodium acetate ethanol solution, wherein the ratio of the amount of sodium hydroxide to aluminum chloride is 3:1. During the dropwise addition process, mechanical stirring was performed at a stirring intensity of 250 rpm, and the dropwise addition was sto...
Embodiment 2
[0072] 5.0 g of magnetic CoFe prepared by co-precipitation method 2 o 4 The particles were evenly dispersed into 90 mL of ethanol solution containing 0.8% sodium acetate, and at 50 °C, 1.28 mL of Tween-80 was slowly added and then refluxed for 12 hours to obtain the surface-modified magnetic CoFe 2 o 4 particle. Magnetic CoFe 2 o 4 The particles were washed three times alternately with ethanol and deionized water. It was dispersed into 100 mL of ethanol solution containing sodium acetate. Lithium chloride and aluminum chloride solution were mixed uniformly according to the ratio of the amount of substances 0.85:1 and dispersed ultrasonically, then added dropwise to the magnetic CoFe at a rate of 300mL / h at the same time 2 o 4 The sodium acetate ethanol solution of the particles, wherein the ratio of the amount of sodium hydroxide to aluminum chloride is 3:1.03. During the dropwise addition, mechanically stir at a stirring intensity of 300 rpm, and stop the dropwise add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com