Method for preparing tazarotene without copper iodide
A technology of tazarotene and cuprous iodide, applied in the field of drug synthesis, can solve the problems of harsh reaction conditions, high recovery cost, difficult to remove, etc., and achieves the effects of short reaction time, high product yield and easy recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
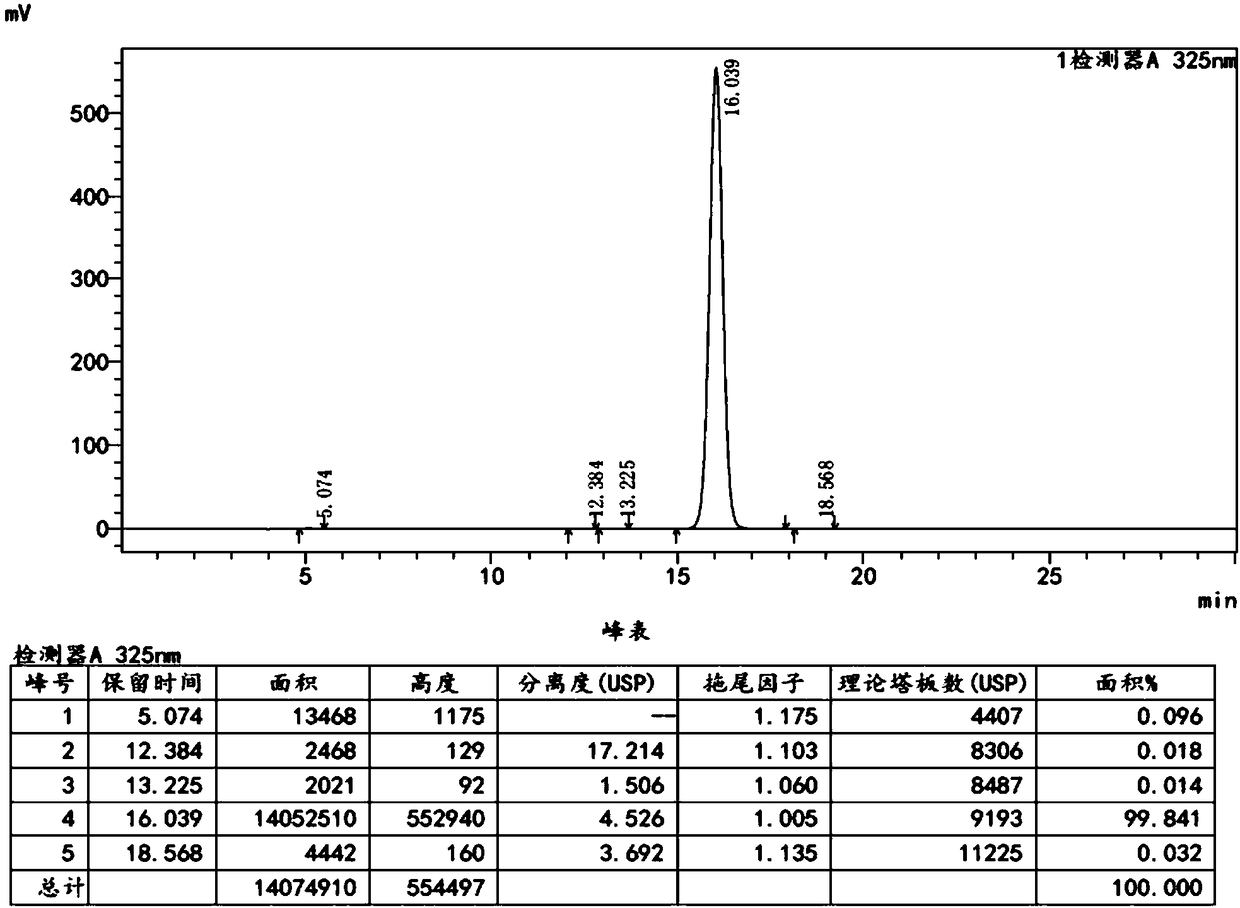
Embodiment 1
[0058] Add 62g of DMF, 0.81g of 10% palladium carbon, and 0.40g of triphenylphosphine into a clean 250mL three-necked flask, stir, and replace with nitrogen. Maintain a weak nitrogen flow and stir for 10-20 minutes. 15.0 g of 4, 4-dimethylbenzothiopyran-6-yl-acetylene, 16.5 g of ethyl 6-chloronicotinate, 12.5 g of potassium acetate, 2.43 g of purified water, and 25 g of DMF were sequentially added. Raise the temperature to 85°C, react for 4 hours, and then monitor by TLC until the spots of 4,4-dimethylbenzothiopyran-6-yl-acetylene disappear (developer dichloromethane:n-hexane=1:6).
[0059]Cool down to room temperature, filter with suction, add 55g of dichloromethane and 470g of drinking water to the filtrate, stir, let stand, and separate the liquids. The combined organic phases were washed twice with 250 g of saturated sodium bicarbonate solution. Separate the liquids, combine the organic phases, and wash in twice with 250 g of saturated sodium chloride solution. Separate...
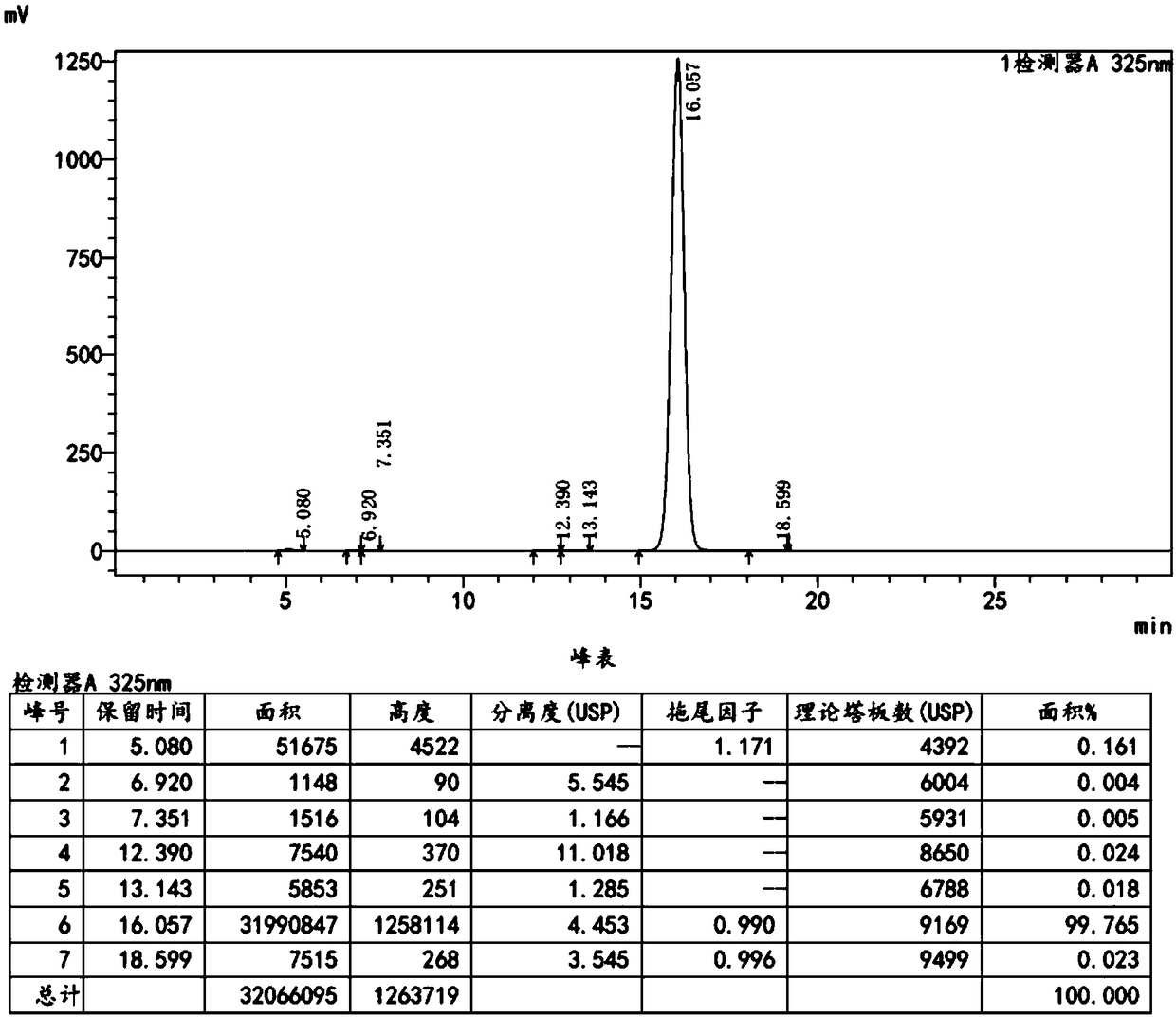
Embodiment 2
[0061] Add 100 g of N-methylpyrrolidone, 0.81 g of 10% palladium carbon, and 0.80 g of triphenylphosphine into a clean 250 mL three-necked flask, stir, and replace with nitrogen. Maintain a weak nitrogen flow and stir for 10-20 minutes. 15.0 g of 4, 4-dimethylbenzothiopyran-6-yl-acetylene, 18.0 g of ethyl 6-chloronicotinate, 12.5 g of potassium carbonate, and 2.43 g of purified water were sequentially added. Raise the temperature to 90°C, react for 5 hours, and then monitor by TLC until the spots of 4,4-dimethylbenzothiopyran-6-yl-acetylene disappear (developing solvent: dichloromethane:n-hexane=1:6).
[0062] Cool down to room temperature, filter with suction, add 55g of dichloromethane and 470g of drinking water to the filtrate, stir, let stand, and separate the liquids. The combined organic phases were washed twice with 250 g of 10% sodium carbonate solution. Separate the layers, combine the organic phases, and wash twice with 250 g of saturated sodium chloride solution. ...
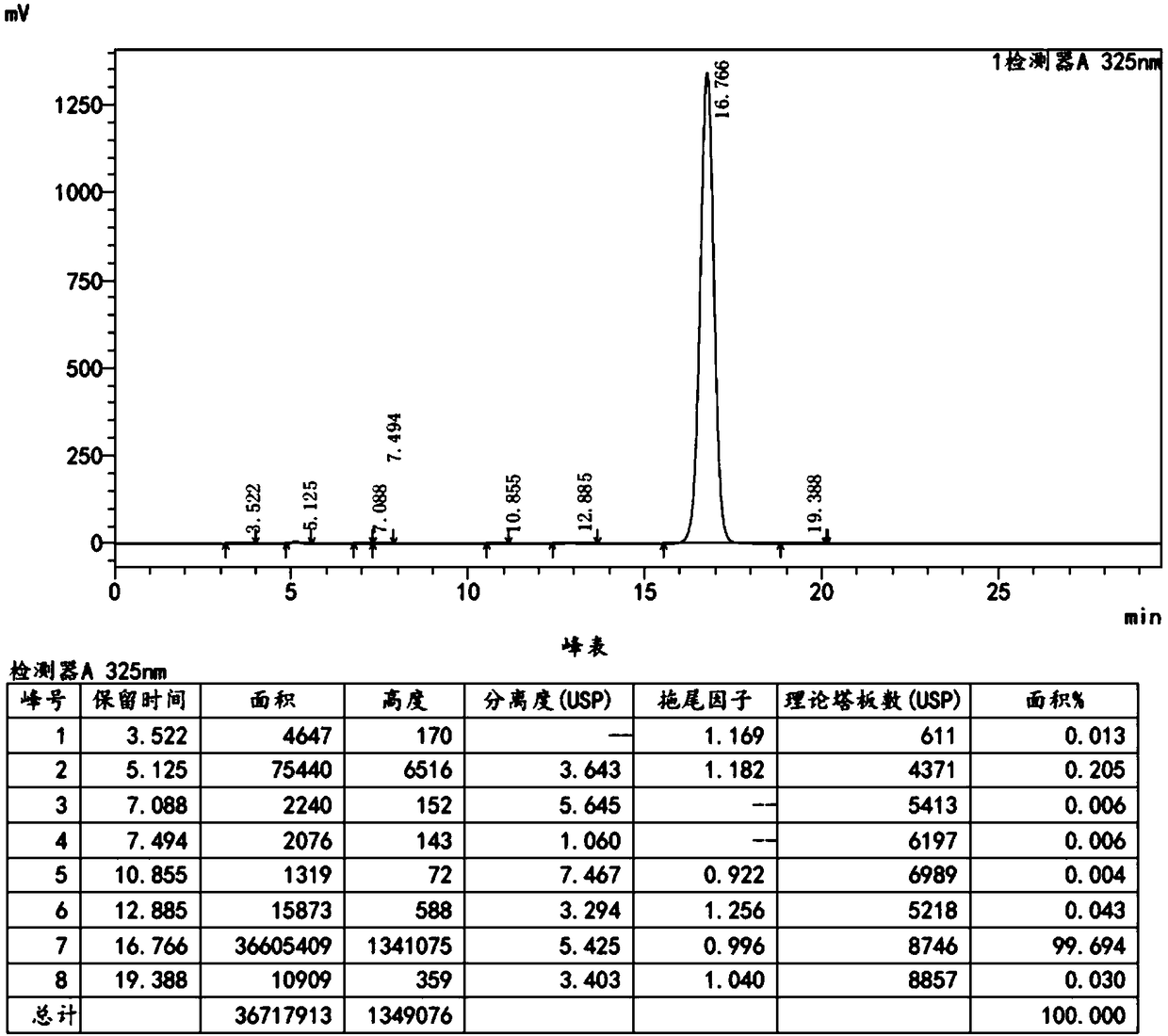
Embodiment 3
[0064] Add 100 g of toluene, 0.81 g of 10% palladium carbon, and 0.40 g of triphenylphosphine into a clean 250 mL three-necked flask, stir, and replace with nitrogen. Maintain a weak nitrogen flow and stir for 10-20 minutes. 15.0 g of 4, 4-dimethylbenzothiopyran-6-yl-acetylene, 15.0 g of ethyl 6-chloronicotinate, 15.0 g of potassium acetate, and 1.62 g of purified water were sequentially added. Raise the temperature to 95°C, react for 6 hours, and then monitor by TLC until the spots of 4,4-dimethylbenzothiopyran-6-yl-acetylene disappear (developing agent: dichloromethane: n-hexane = 1:6).
[0065] Cool down to room temperature, filter with suction, add 55g of dichloromethane and 470g of drinking water to the filtrate, stir, let stand, and separate the liquids. The combined organic phases were washed twice with 250 g of 10% sodium carbonate solution. Separate the layers, combine the organic phases, and wash twice with 250 g of saturated sodium chloride solution. Separate the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com