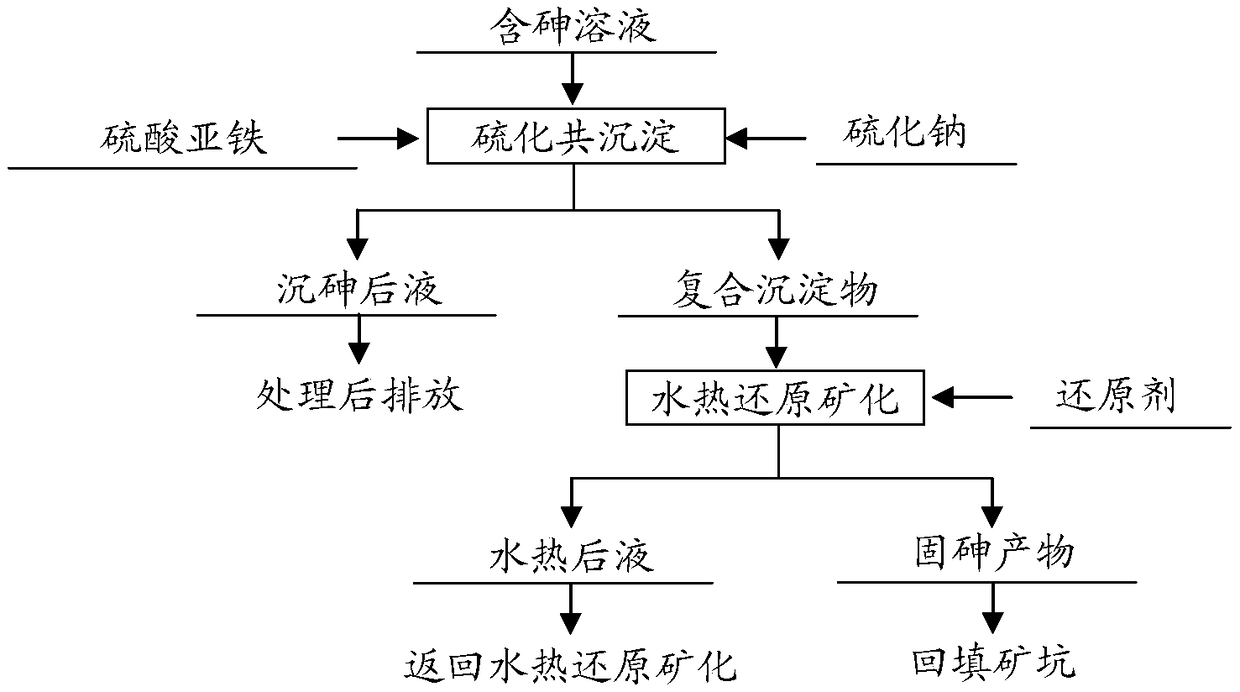
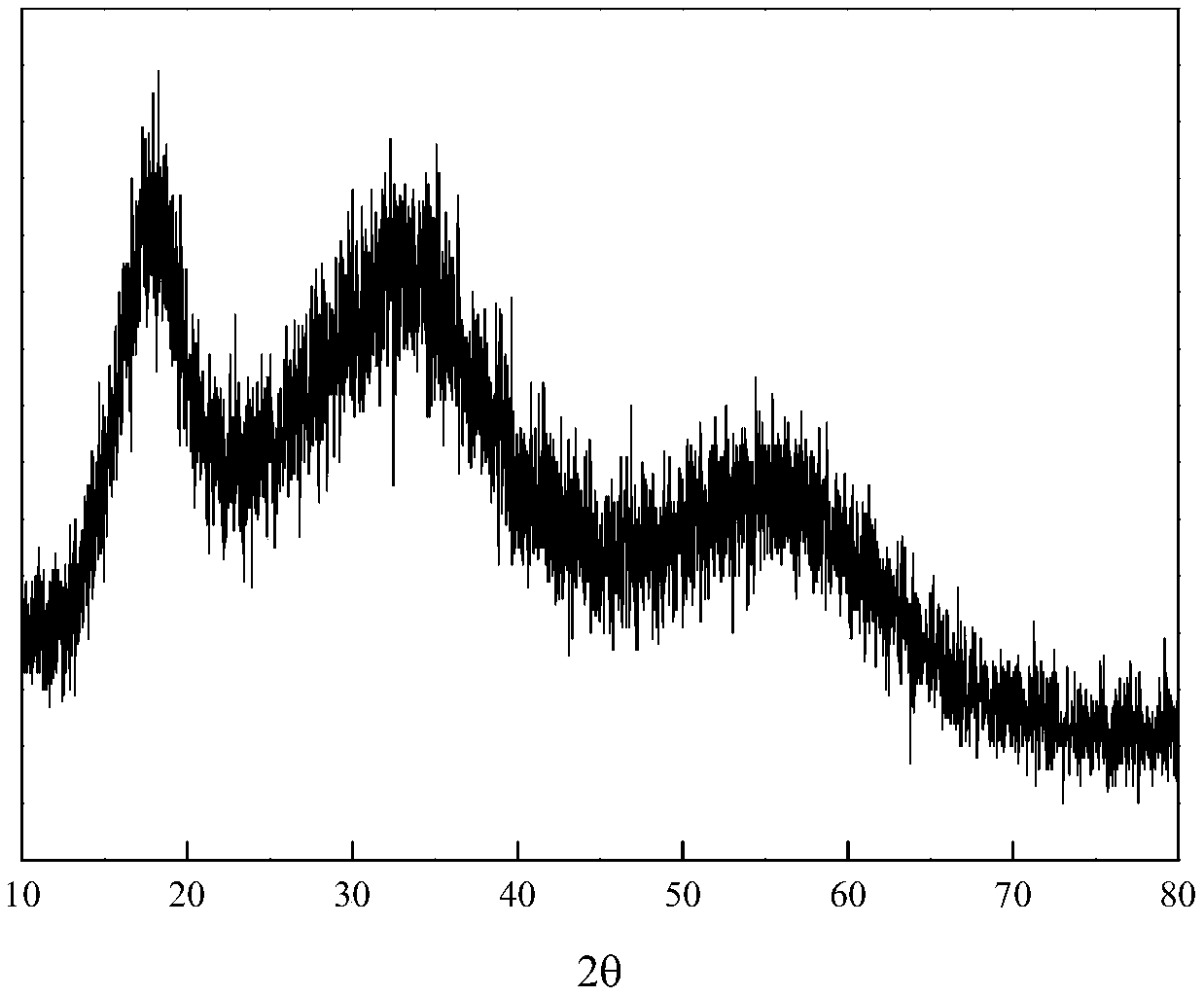
Method for carrying out hydrothermal reduction on mineralized synergistic fixed arsenic
A hydrothermal and sodium sulfide technology, applied in chemical instruments and methods, arsenic sulfide, reduced water/sewage treatment, etc., can solve the problems of high leaching rate of solidified body, poor durability, low arsenic content, etc., and reduce storage space demand, reduce dissolution toxicity, and stabilize technical indicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The arsenic concentration in the arsenic-containing solution is 11000 mg / L, the pH is 0.3, and ferrous sulfate, concentrated sulfuric acid, sodium hydroxide, sodium sulfide and glucose are all industrial grade reagents. Add ferrous sulfate to the arsenic-containing solution, control the molar ratio of Fe / As in the solution to 0.5 / 1, and then use a sodium sulfide solution with a concentration of 100g / L to control the molar ratio of sodium sulfide to arsenic and iron to 1.5 / 1, respectively And 1.0 / 1, while adding concentrated sulfuric acid to adjust the solution pH=4.5, react at 60℃ for 60min, after the reaction, adopt vacuum filtration method to separate liquid and solid; liquid-solid according to the ratio of water volume L to mass of composite precipitate in kg Add water to slurry at a ratio of 3~10 / 1, then add 2.0% glucose of the composite precipitate mass as a reducing agent, add the mixed slurry to a stainless steel high-pressure reactor, and pour nitrogen into the re...
Embodiment 2
[0028] The arsenic concentration in the arsenic-containing solution is 12,500 mg / L, the pH is 0.1, and ferrous sulfate, concentrated sulfuric acid, sodium hydroxide, sodium sulfide and glucose are all industrial grade reagents. Add ferrous sulfate to the arsenic-containing solution, control the molar ratio of Fe / As in the solution to 0.7 / 1, and then use a sodium sulfide solution with a concentration of 100g / L to control the molar ratio of sodium sulfide to arsenic and iron to 1.7 / 1, respectively At the same time, add concentrated sulfuric acid to adjust the pH of the solution to pH=4.0, and react at 60℃ for 60min. After the reaction, vacuum filtration is used for liquid-solid separation; the liquid-solid ratio of the water volume L to the composite precipitate mass kg Add water to slurry at a ratio of 3~10 / 1, then add 2.5% glucose of composite precipitate mass as a reducing agent, add the mixed slurry to the stainless steel high-pressure reactor, and pour nitrogen into the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com