Method for synthesizing alpha-amino ketone derivatives
An amino ketone and derivative technology, applied in the field of organic synthesis, can solve the problems of complex preparation of methylene azetidine, complex preparation of starting materials, limited substrate scope, etc., and achieves reaction regioselectivity and The effect of high chemical selectivity, improved atom utilization, and high atom utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The reaction synthesis steps and product structural formula of the present embodiment are as follows:
[0084]
[0085] The specific operation steps of this embodiment are as follows:
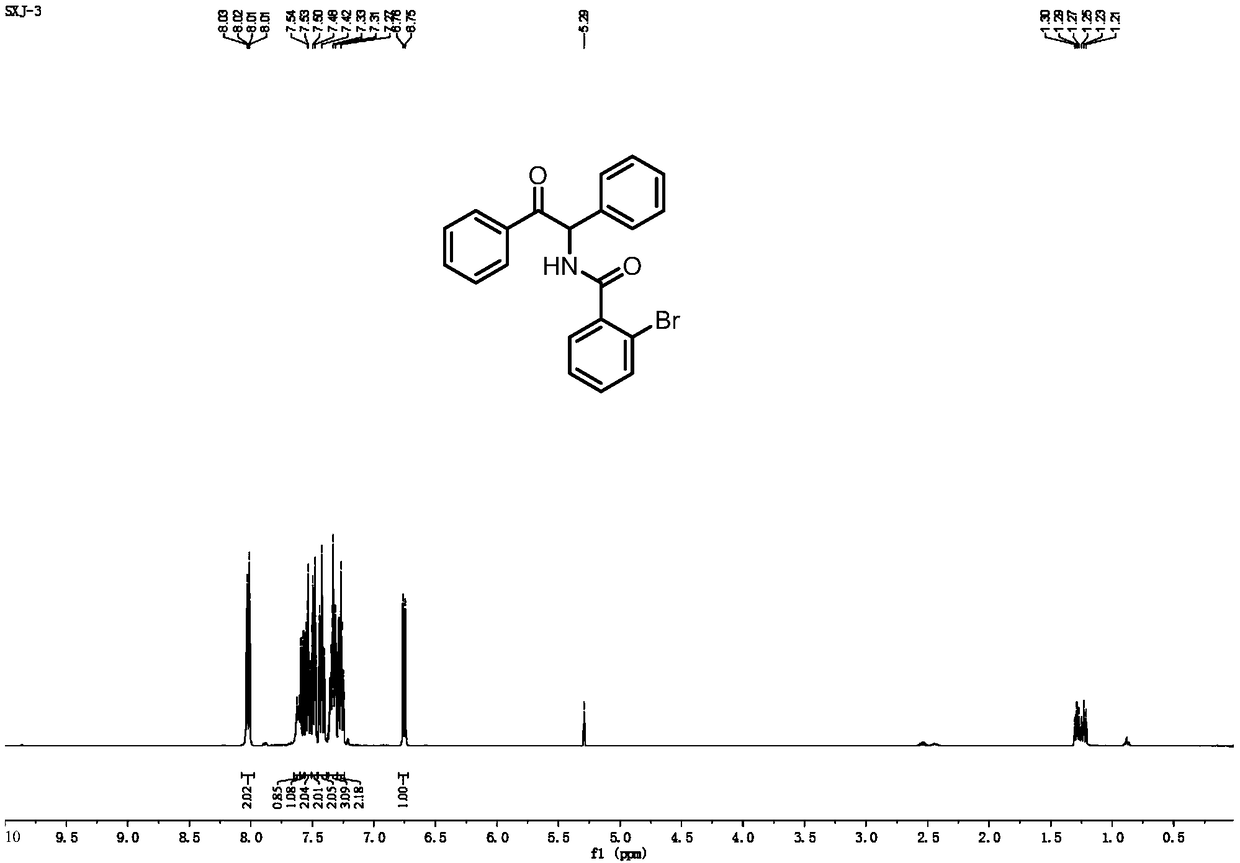
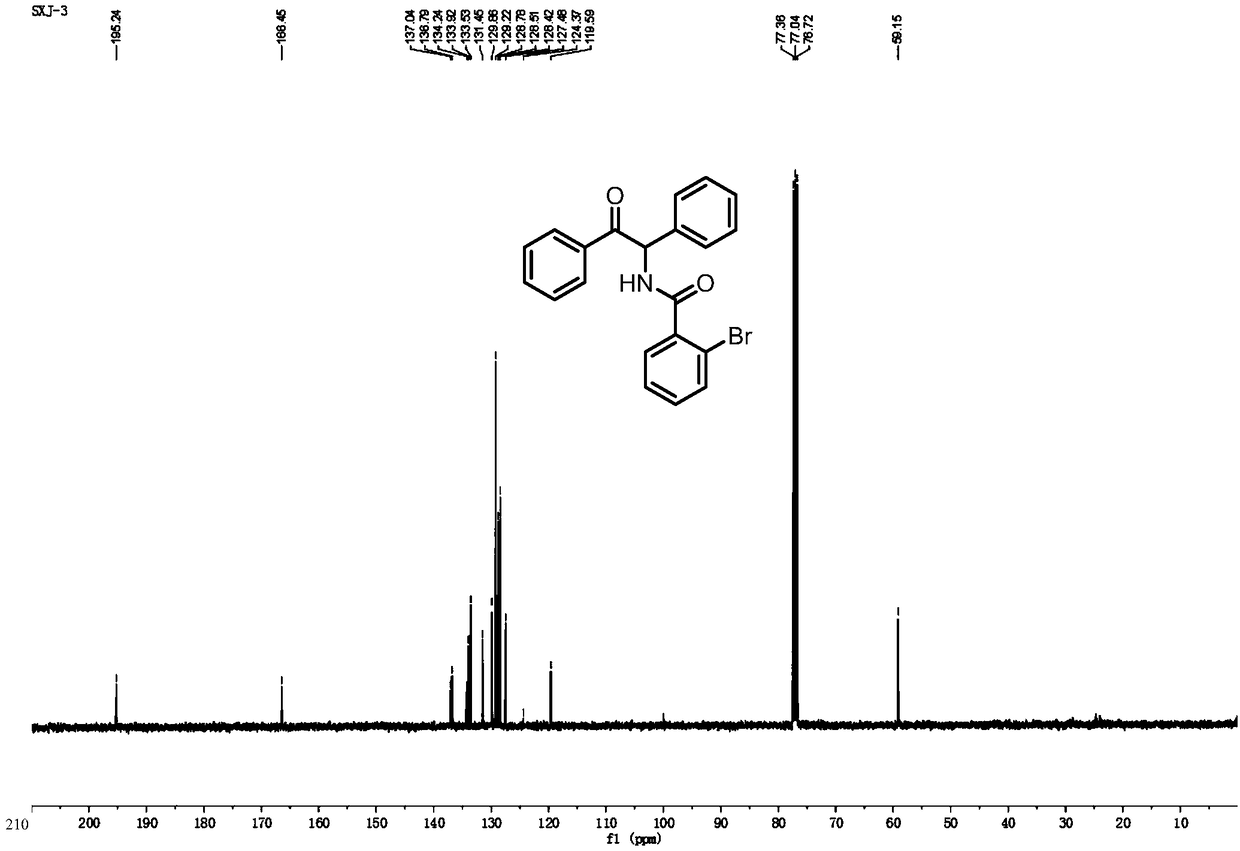
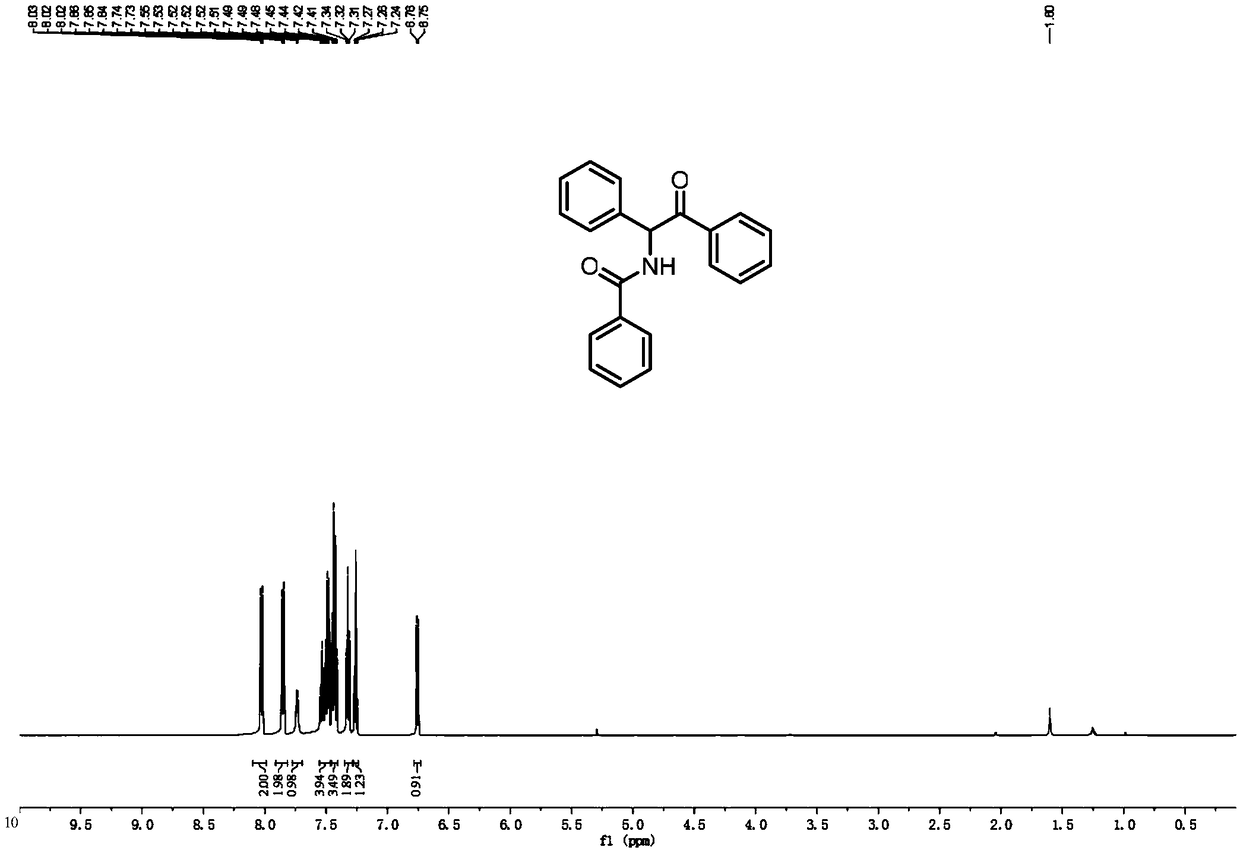
[0086] Add Pd(OAc) to the reaction vessel 2 (2.8mg, 0.0125mmol), chloro[1,3-bis(2,6-diisopropylphenyl) imidazol-2-ylidene] silver (6.6mg, 10mol%), DMAP (1equiv), Li 2 CO 3 (1equiv), carboxylic acid compound 1a (25.1mg, 0.125mmol), 2H-aziridine compound 2a (24.2mg, 0.125mmol), dioxane (2mL), heated to 110°C, and reacted for 18 hours. The reaction solution was cooled to room temperature, and after the solvent was removed under reduced pressure, the corresponding α-aminoketone derivative (3aa) was separated by column chromatography, and its structure was determined by NMR and high-resolution mass spectrometry, see figure 2 , image 3 and Figure 10 As shown, the yield can reach 92%.
[0087] The NMR data of the α-aminoketone derivative 3aa obtained in this embodiment are as follow...
Embodiment 2
[0090] The reaction synthesis steps and product structural formula of the present embodiment are as follows:
[0091]
[0092] The specific operation steps of this embodiment are as follows:
[0093] Add Pd(OAc) to the reaction vessel 2 (2.8mg, 0.0125mmol), chloro[1,3-bis(2,6-diisopropylphenyl) imidazol-2-ylidene] silver (6.6mg, 10mol%), DMAP (1equiv), Li 2 CO 3 (1equiv), carboxylic acid compound 1a (251.3mg, 1.25mmol), 2H-aziridine compound 2a (241.6mg, 1.25mmol), dioxane (2mL), heated to 110°C, and reacted for 18 hours. The reaction solution was cooled to room temperature, and after the solvent was removed under reduced pressure, the corresponding α-aminoketone derivative (3aa) was separated by column chromatography, and its structure was determined by NMR and high-resolution mass spectrometry, see figure 2 , image 3 and Figure 10 As shown, the yield can reach up to 30%.
Embodiment 3
[0095] The reaction synthesis steps and product structural formula of the present embodiment are as follows:
[0096]
[0097] The specific operation steps of this embodiment are as follows:
[0098] Add Pd(OAc) to the reaction vessel 2 (2.8mg, 0.0125mmol), chloro[1,3-bis(2,6-diisopropylphenyl) imidazol-2-ylidene] silver (6.6mg, 10mol%), DMAP (1equiv), Li 2 CO 3 (1equiv), carboxylic acid compound 1a (20.1mg, 0.1mmol), 2H-aziridine compound 2a (24.6mg, 0.125mmol), dioxane (2mL), heated to 110°C, and reacted for 18 hours. The reaction liquid was cooled to room temperature, and after the solvent was removed under reduced pressure, the corresponding α-amino ketone derivative (3aa) was obtained by column chromatography separation. Mass spectrometry was used to determine its structure, see figure 2 , image 3 and Figure 10 As shown, the yield can reach up to 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com