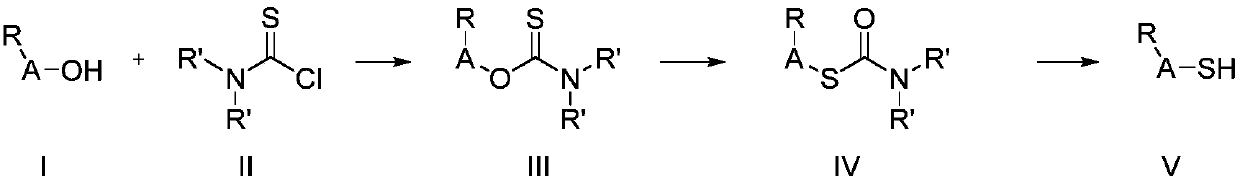
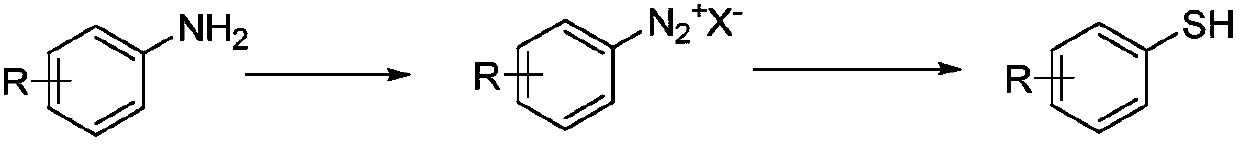
Method for preparing substituted thiophenol and heterocyclic thiophenol by continuous flow reactor
A reactor, thiophenol technology, applied in the preparation of mercaptans, the formation/introduction of mercapto/thioether groups, and organic chemistry, etc., can solve the problems of limitation, long reaction steps, and less amount of three wastes, and reduce energy consumption. , to avoid flying temperature, the effect of reducing the amount of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] a. Add 6kg of ethylene dichloride and 1.63kg of tetramethylthiuram disulfide (TMTD) in the reaction flask, heat up to 60 degrees, add 700g of sulfuryl chloride dropwise, and keep the internal temperature between 60-65 degrees, After dripping and keeping warm for 2 hours, 200-300 g of dichloroethane was removed under reduced pressure, then cooled to room temperature, and the solid was removed by filtration to obtain the intermediate II solution, which was directly used in the next reaction.
[0048] b. Add 1221.2g 3-formylphenol in the reaction flask, add dropwise 1333.3g 30% liquid caustic soda, and control the temperature at the same time not to exceed 30°C. After dropping, slowly add the above-mentioned intermediate II solution to the system, and maintain the reaction temperature at 25°C. -35°C, keep the reaction for 2 hours after dropping, and adjust the pH to 6-7 with concentrated hydrochloric acid. The reaction solution was suction filtered, the filter ...
Embodiment 2
[0052]
[0053] a. With embodiment 1a.
[0054] b. Add 1221.2g 3-formylphenol, 1062.5g triethylamine, 3662.6g dichloroethane to the reaction flask, stir to dissolve, slowly add the above-mentioned intermediate II solution into the system, and maintain the reaction temperature at 25-35 ℃, after dripping, keep it warm for 2 hours, adjust the pH to 6-7 with concentrated hydrochloric acid, separate the liquid, desolventize the organic phase to obtain the crude product of intermediate II, recrystallize the crude product with ethanol, dry under reduced pressure to obtain intermediate III 2124.0g, yield 89.5%, liquid phase content 98.5%.
[0055] c. Add 2124.0g of intermediate III and 1000g of diphenyl ether into the reaction bottle, stir to dissolve, inject into the reaction pipeline by syringe pump (pipeline length 120m, Φ6*1mm), feed rate 150mL / min, oil temperature 255℃ ~275°C, hot water is cooled and flowed into another reaction bottle containing 1100g of 30% liquid caustic s...
Embodiment 3
[0058]
[0059] a. Add 6kg of ethylene dichloride and 1.63kg of tetramethylthiuram disulfide (TMTD) in the reaction flask, heat up to 60 degrees, add 700g of sulfuryl chloride dropwise, and keep the internal temperature between 60-65 degrees, After dripping and keeping warm for 2 hours, 200-300 g of dichloroethane was removed under reduced pressure, then cooled to room temperature, and the solid was removed by filtration to obtain the intermediate II solution, which was directly used in the next reaction.
[0060] b. Add 1451.6g 5-hydroxyisoquinoline in the reaction flask, add dropwise 1333.3g 30% liquid caustic soda, and control the temperature at the same time not to exceed 30°C. After dropping, slowly add the above-mentioned intermediate II solution to the system to maintain the reaction temperature 25-35°C, keep the reaction for 2 hours after dropping, adjust the pH to 6-7 with concentrated hydrochloric acid. The reaction solution was filtered with suction, the filter c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com