Arylamine derivative, and preparation method and application thereof
A technology of derivatives and aromatic amines, applied in the field of organic electroluminescent devices, to achieve high luminous efficiency, low driving voltage, and improved performance parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] The application also provides the preparation method of the arylamine derivative, specifically:
[0066] reacting the compound having the structure of formula (IV) with the compound having the structure of formula (V) under the action of a catalyst and an alkali metal salt to obtain an arylamine derivative having the structure of formula (I);
[0067]
[0068]
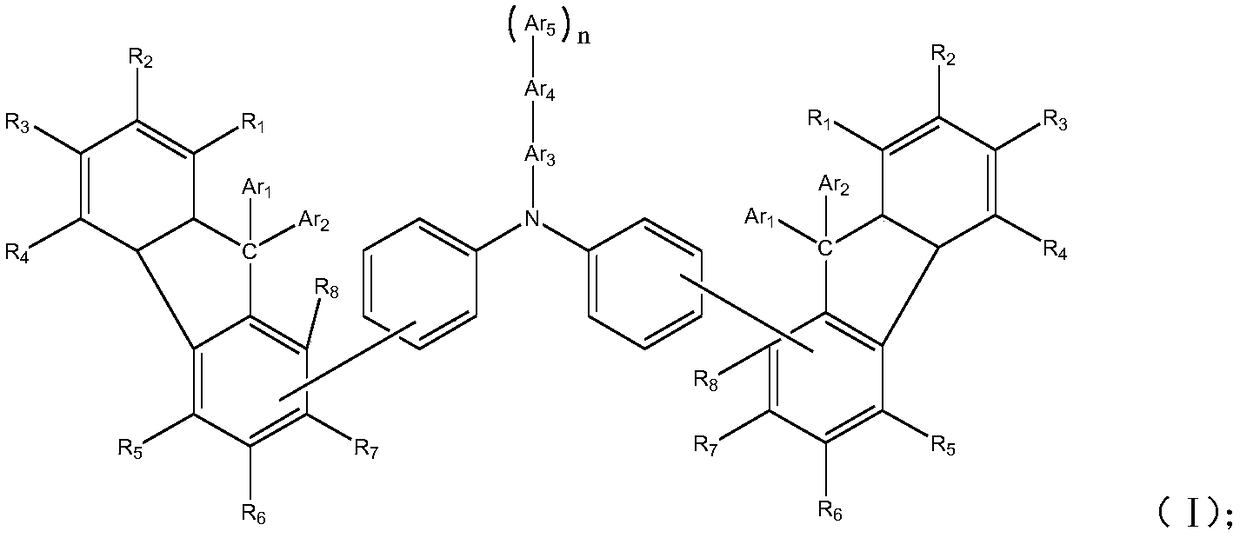
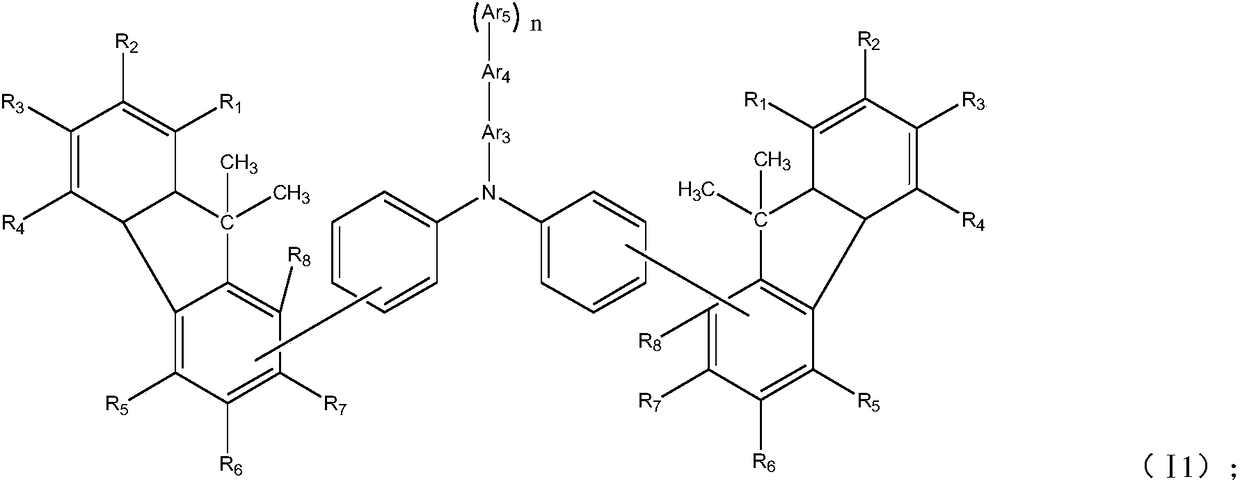
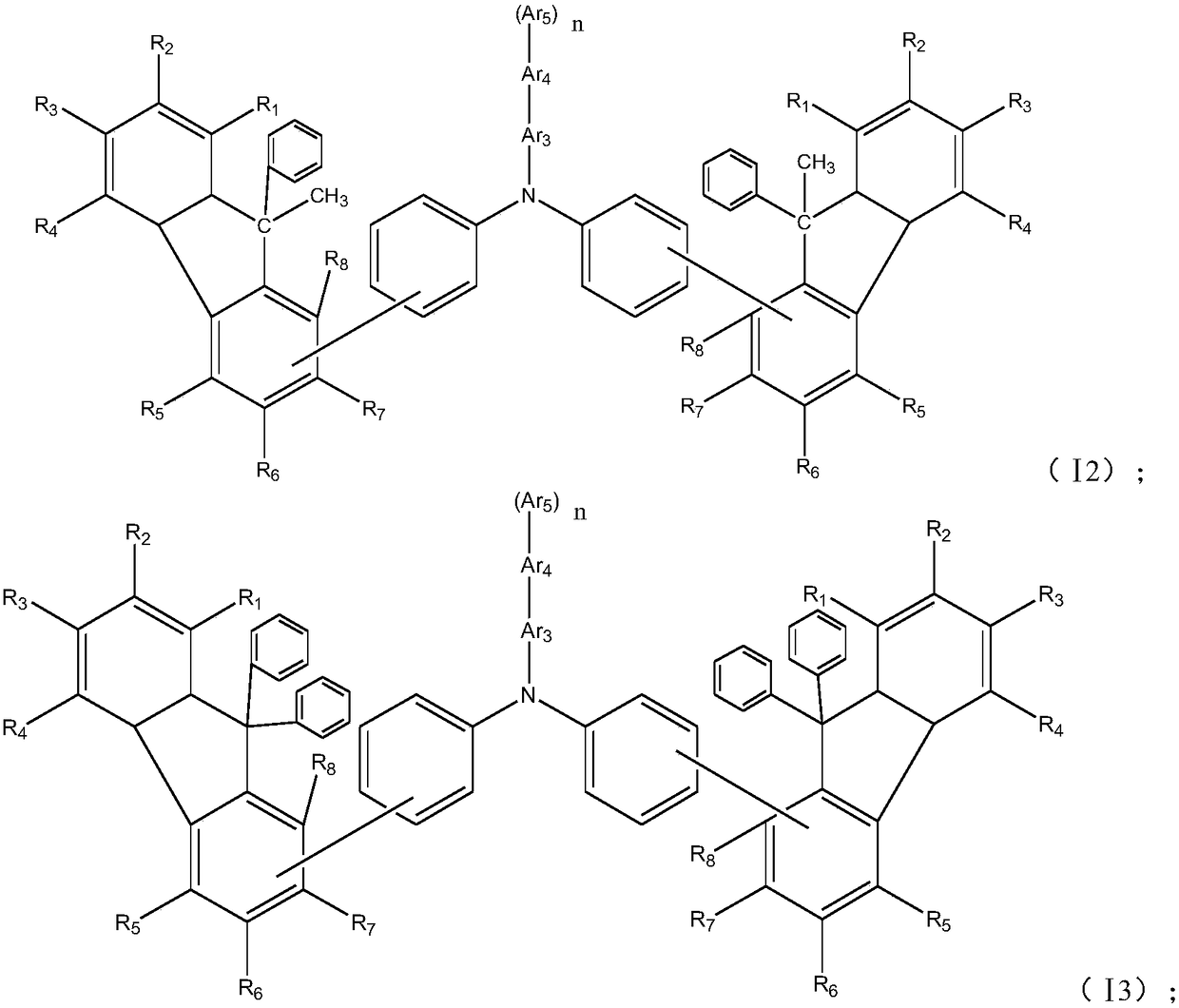
[0069] Among them, R 1 ~R 8Each independently selected from hydrogen, deuterium, halogen, cyano, hydroxyl, nitro, substituted or unsubstituted C1~C20 alkyl, substituted or unsubstituted C6~C30 aryl, substituted or unsubstituted C2~ C30 heteroaryl, substituted or unsubstituted C1-C20 alkoxy, substituted or unsubstituted C6-C20 aryloxy, substituted or unsubstituted C3-C40 silyloxy, substituted or unsubstituted C1-C20 acyl, substituted or unsubstituted C2-C20 alkoxycarbonyl, substituted or unsubstituted C2-C20 acyloxy, substituted or unsubstituted C2-C20 amido, substituted or unsubstituted C2-C20 alkoxycar...
Embodiment 1
[0087] In a nitrogen atmosphere, 10.0g (11.7mmol) of intermediate M-3-1-1, 3.82g (15.88mmol) of 2-chloro-4-phenylquinazoline and 0.15g (0.13mmol) of (Triphenylphosphine) palladium is put into a flask and dissolved in a mixed solvent of 250ml of toluene, ethanol and water (volume ratio 3:1:1); then, the aqueous solution of potassium carbonate of 7.3g (52.92mmol) is added to the above The reactant was then refluxed and stirred for 12 hours; after the reaction, the reactant was extracted with ethyl acetate, and the extracted product was dried with magnesium sulfite and filtered; then, the filtered product was concentrated under reduced pressure, and silica gel column chromatography (developing agent ratio : Petroleum ether / dichloromethane=3:1) purify concentrated product, obtain the compound 14 (yield=82%) of 9.05g, mass spectrum: theoretical value is 833.38; Measured value is 833.40; Above-mentioned reaction process is specifically as follows:
[0088]
Embodiment 2
[0090] The preparation method is the same as in Example 1, the difference is that 2-chloro-4-phenylquinazoline is replaced by 4-bromobiphenyl, and finally 90g of compound 21 (yield=87%) is obtained, and the mass spectrum: the theoretical value is 781.37; measured 781.39. The reaction process is specifically as follows:
[0091]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com