Staphylococcus aureus inactivated vaccine for cow mastitis and preparation method thereof
A technology for dairy cow mastitis and staphylococcus, applied in vaccines, veterinary vaccines, antibacterial drugs, etc., can solve the problems of limited immune protection efficacy and antigen loss of inactivated whole bacteria vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
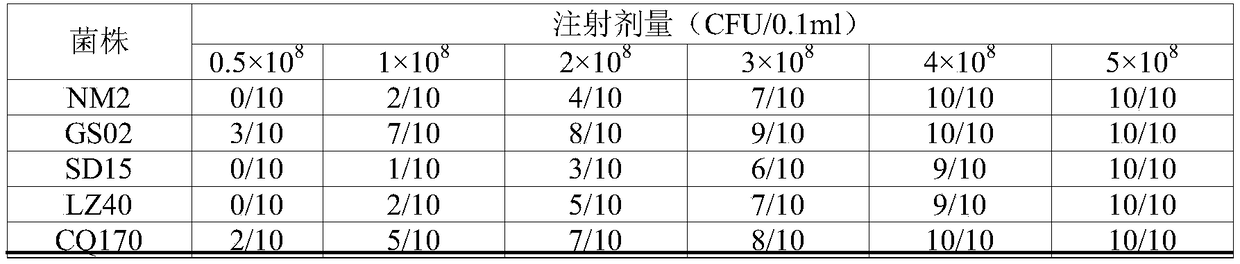
[0133] Example 1: Screening and preservation of inactivated bacterial vaccine strains and bacterial strains for testing
[0134] 1. Screening and determination of prevalent strains of Staphylococcus aureus infected with cow mastitis
[0135] Nearly 30,000 milk samples for clinical mastitis were collected from 90 medium and large dairy farms covering 30 provinces and cities in my country, including Inner Mongolia, Chongqing, Guangdong, Heilongjiang, Gansu, Hebei, Shandong, and Xinjiang, and were separated and tested. To screen and determine the dominant prevalent strains of Staphylococcus aureus in dairy cow mastitis. Specific steps are as follows:
[0136] 1. Collection of milk samples from dairy cows
[0137] Select dairy cows with clinical symptoms of mastitis (or suspected subclinical mastitis), first scrub the udders with warm water, then 0.2% bromogeramine, and finally wipe the nipples with 70% alcohol. Samplers wipe and disinfect their fingers at the same time. Squeez...
Embodiment 2
[0171] Embodiment 2: Construction of recombinant SEB protein Escherichia coli BL21 strain
[0172] 1. Optimization of SEB sequence
[0173] The Staphylococcus aureus enterotoxin B (SEB) gene sequence is optimized, the optimized SEB gene sequence is shown in sequence 1 in the sequence listing, and the amino acid sequence of the encoded SEB protein is shown in sequence 2 in the sequence listing. The specific steps are as follows: According to GenBank (AB479118.1) and the SEB gene sequence of the reported site-directed mutation human vaccine, obtain its spatial three-dimensional structure model to mutate two amino acids at the binding residue of SEB and MHCII molecule and one amino acid on the TCRβ chain to reduce the The virulence of SEB, its protein antigen conservation, immunogenicity and antigen neutralization epitope characteristics were preserved. Point mutations were carried out at the 46th, 90th and 95th SEB hydrophobic binding region, polar binding region and disulfide ...
Embodiment 3
[0180] Embodiment 3: the construction of recombinant H1α protein Escherichia coli BL21 strain
[0181] 1. Optimization of Hlα sequence
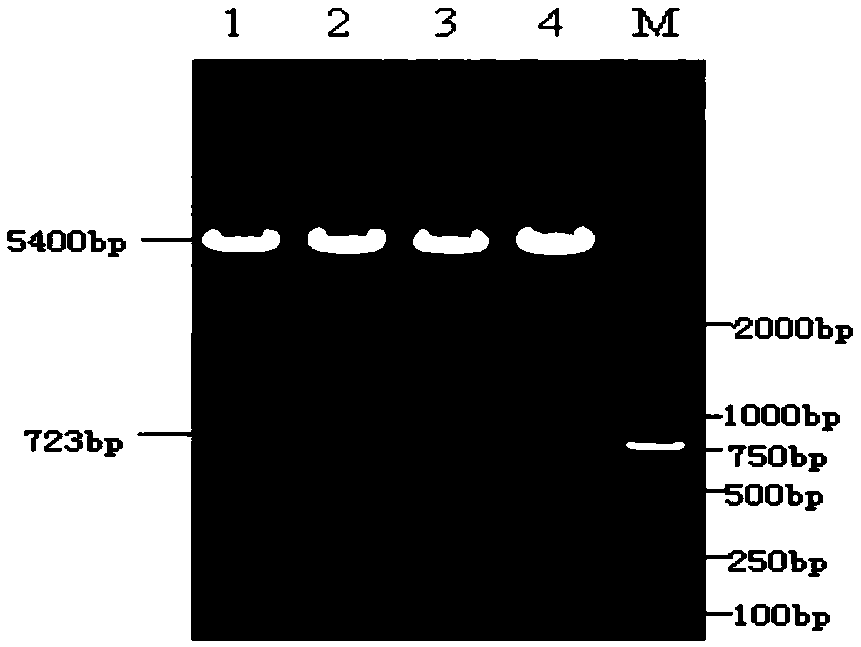
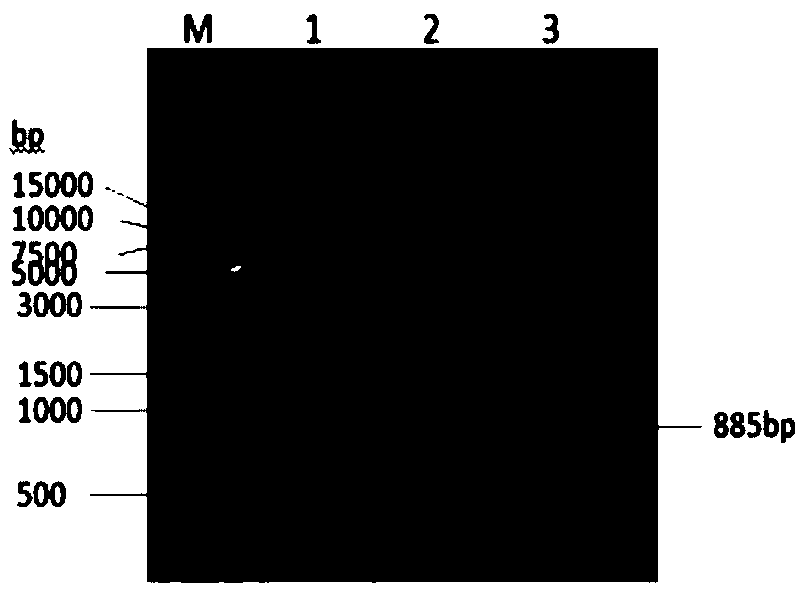
[0182] The Staphylococcus aureus alpha hemolysin (Hlα) gene sequence is optimized, the optimized Hlα gene sequence is shown in sequence 3 in the sequence listing, and the amino acid sequence of the encoded Hlα protein is shown in sequence 4 in the sequence listing. The specific steps are as follows: design the Hlα gene sequence according to GenBank (X55185.1) and published literature, first of all, in view of the strong toxicity of Hlα, the active site H35 is mutated, and leucine Leu is used instead of histidine His . At the same time, in order to increase the expression of soluble Hlα protein, the base codon of the Hlα gene sequence was optimized to Escherichia coli preferred codons with a length of 885 bp using vector NTI advance 11 software. The optimized Hlα base sequence was synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com