Xanthene fluorescent probe and preparation method and application thereof
A fluorescent probe, xanthene technology, applied in the field of fluorescent probes, can solve problems such as limiting the application of fluorescent probes, and achieve the effects of easy promotion and application, good selectivity, and good permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A preparation method of xanthene fluorescent probe, comprising the following steps:
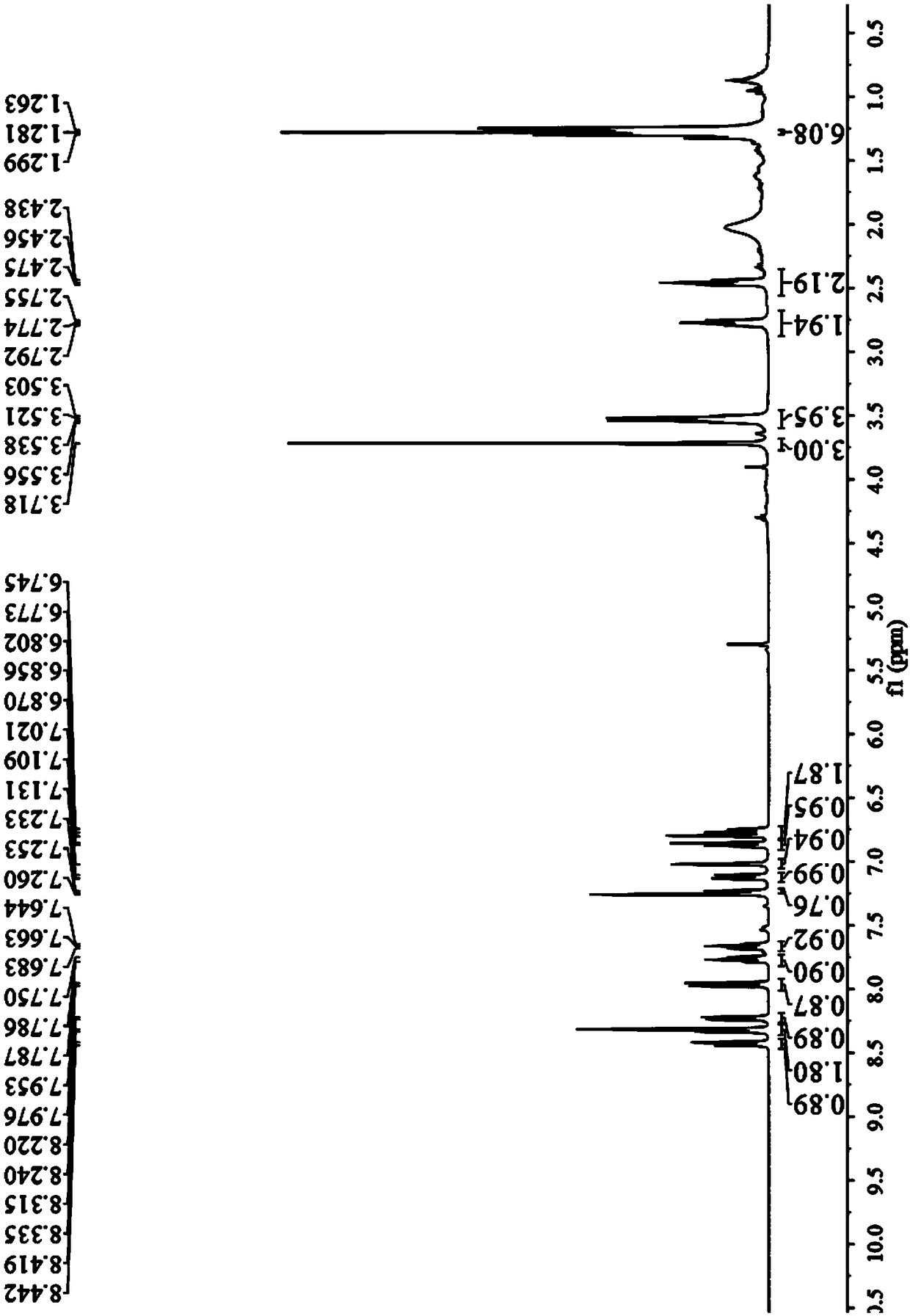
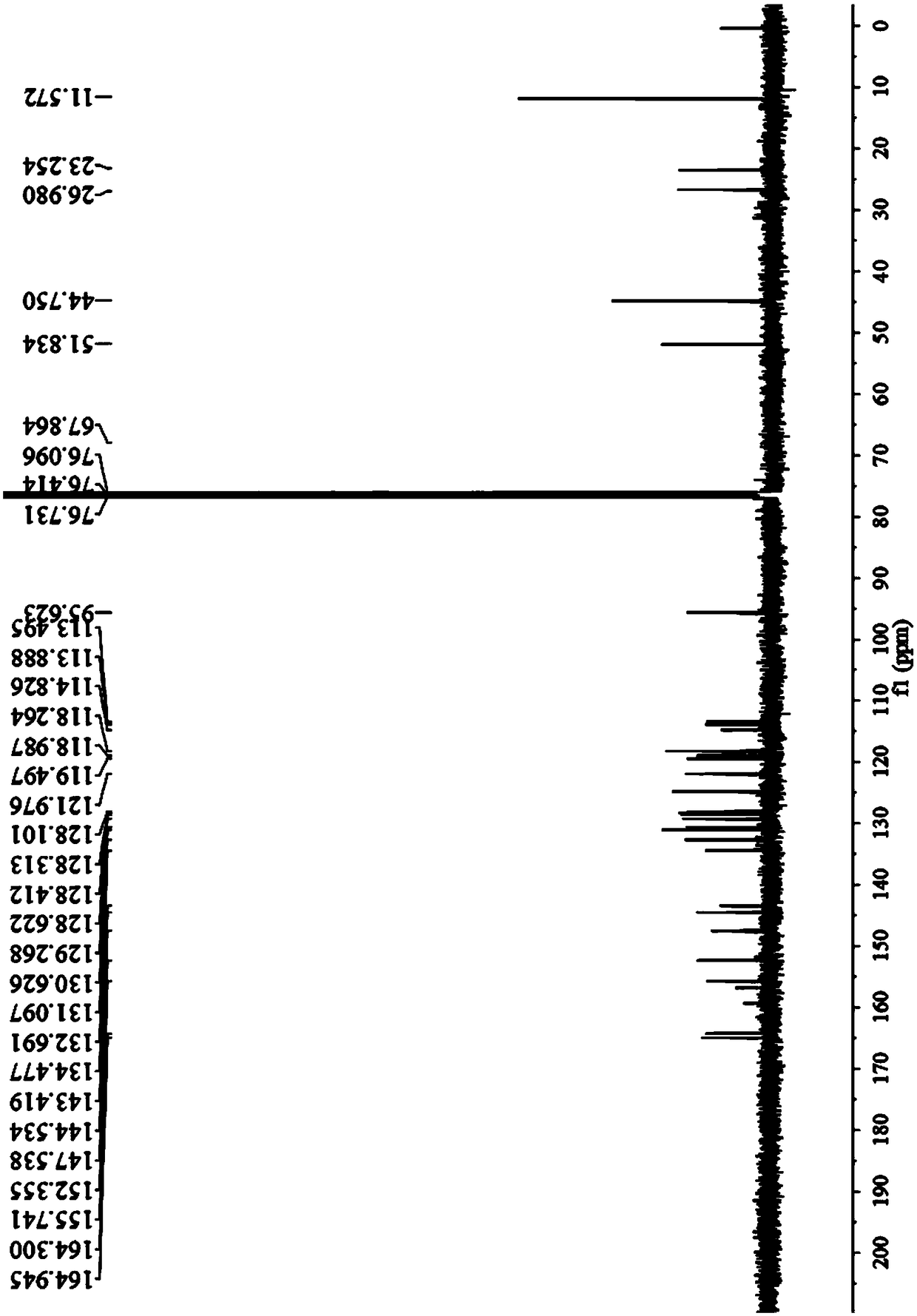
[0051](1) Preparation of the compound of general formula (III): Add 4-diethylamino ketoacid (1.72g, 5.5mmol) into 10mL methanesulfonic acid, heat to dissolve at 100°C, then add 6-amino-1 , 2,3,4-tetrahydro-1-naphthone (0.81g, 5mmol), continued heating and stirring for 4 hours, then stopped the reaction. After the reaction mixture was cooled to room temperature, the reactant was slowly added to a beaker filled with ice cubes under stirring, and then 10mL perchloric acid (70%) was slowly added dropwise, and a brown-red solid precipitate slowly appeared in the beaker, and placed After standing in the refrigerator for a period of time, suction filtration was performed and dried to obtain 2.24 g of a purple solid with a yield of 83%. 1 H-NMR (400MHz, MeOD), δ8.21 (d, J = 7.7Hz, 1H), 8.08 (dd, J = 8.8, 4.3Hz, 1H), 7.74 (t, J = 7.4Hz, 1H), 7.68 (t,J=7.5Hz,1H),7.29(d,J=7.4Hz,1H),7.05(s,1H),7...
Embodiment 2
[0058] Preparation of a kind of xanthene fluorescent probe and some analyte stock solutions
[0059] A certain amount of the xanthene-based fluorescent probe prepared in Example 1 was dissolved in chromatographic-grade acetonitrile solvent to prepare a 0.5 mM stock solution and store it in a refrigerator. A certain amount of GSH was dissolved in twice-distilled water to prepare a 35 mM stock solution and stored in a refrigerator. Other analytes include amino acids (Ala, Arg, His, Lys, Met, Ser, Tyr), inorganic salts (Al(NO 3 ) 3 , CaCl 2 、CuSO 4 , MgCl 2 , NaBr, NaCl, Na 2 S) and other biological thiols (Cys, Hcy) were prepared into a 10 mM stock solution using twice distilled water as a solvent and stored in a refrigerator. A certain amount of N-ethylmaleimide (NEM) was dissolved in double distilled water to prepare a 50 mM stock solution and stored in a refrigerator. A certain amount of N-acetyl-L-cysteine (NAC) was dissolved in double distilled water to prepare a 1...
Embodiment 3
[0063] Selectivity and anti-interference experiments of xanthene-based fluorescent probes:
[0064] Using the solution prepared in Example 2, the mixed solvent (PBS: CH 3 CN=8:2, pH=7.4) were added different amino acids (Ala, Arg, His, Lys, Met, Ser, Tyr, 0.5mM), cations (Al 3+ , Ca 2+ 、Cu 2+ , Mg 2+ 、Na + ,0.5mM), anion (Cl - , Br, SO 4 2 , S 2- ,0.5mM), other biological thiols (Cys, Hcy,0.5mM), GSH (7mM) measure the fluorescence intensity at a wavelength of 630nm. Depend on Figure 6 It can be seen that except for the addition of Cys and GSH, the fluorescence intensity is enhanced by about 20 times and 100 times, and other analytes hardly cause changes in the fluorescence intensity. Further, the fluorescence spectra of Cys at six concentrations of 0, 20, 50, 100, 200 and 500 μM were made. Such as Figure 7 As shown, when the Cys concentration is 0-50 μM, the fluorescence intensity hardly changes. When the Cys concentration is 100-500 μM, the fluorescence intensit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com