Dual-emitting cellulose-based fluorescent material, preparation method and applications thereof
A fluorescent material and cellulose-based technology, which can be used in luminescent materials, luminescent coatings, fluorescence/phosphorescence, etc., can solve the problems of limited practical application, inability to use materials alone, and low measurement accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Preparation of Dual Emission Cellulose-Based Fluorescent Materials by Twice Esterification
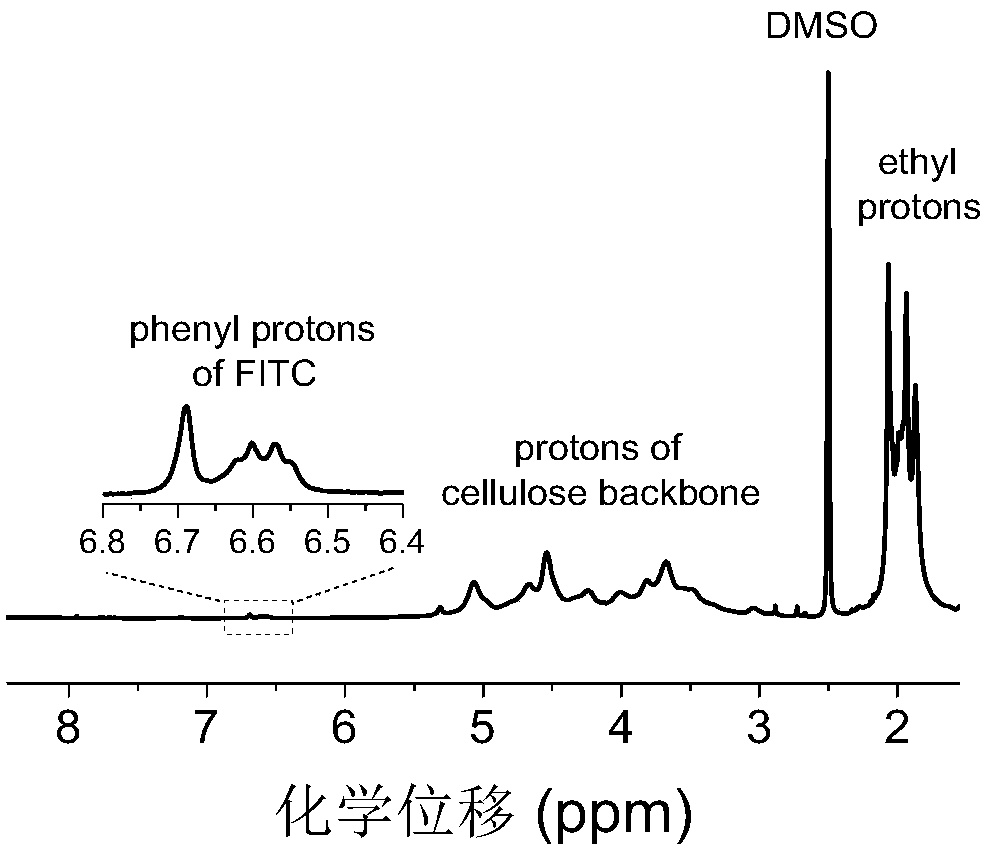
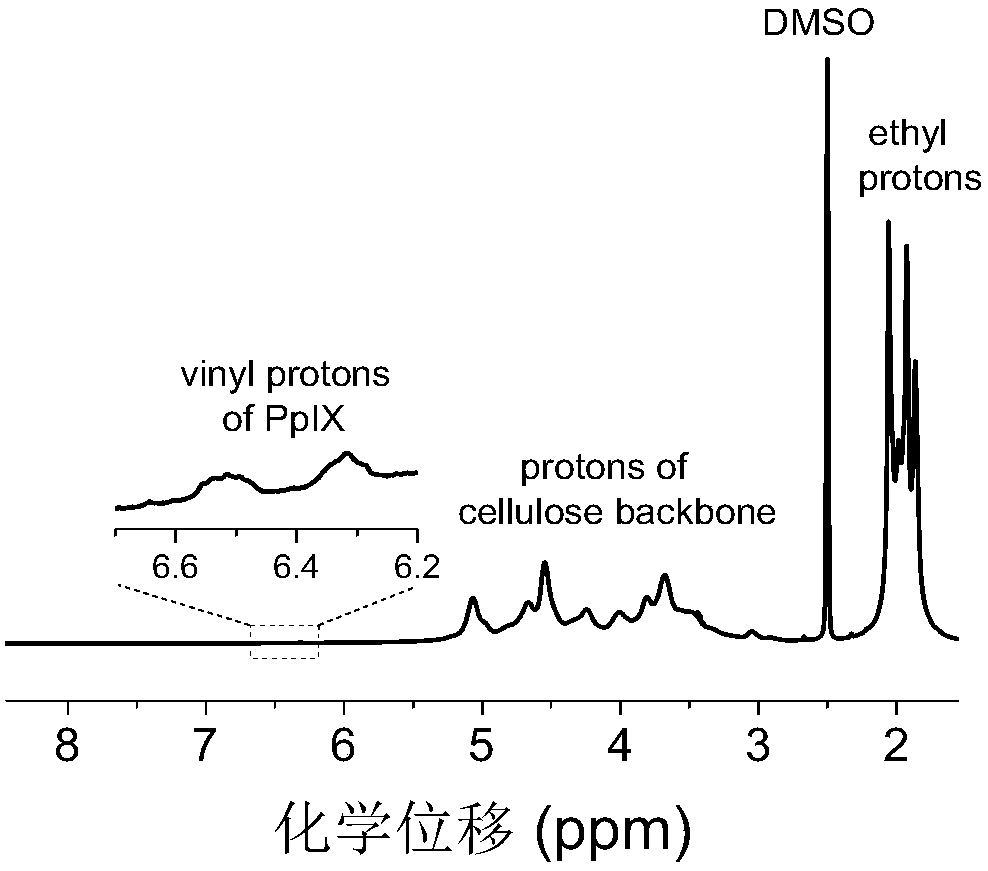
[0085] Weigh 1.9g cellulose acetate butyrate and 0.016g fluorescein isothiocyanate to carry out esterification reaction under the catalytic conditions of dibutyltin dilaurate (esterification reaction temperature is 100°C; esterification reaction time is 4h) , the obtained product was precipitated, washed and dried, then dissolved again, and subjected to an esterification reaction with 0.11 g of protoporphyrin under the catalysis of carbonyldiimidazole (the esterification reaction temperature was 80° C.; the esterification reaction time was 20 h).
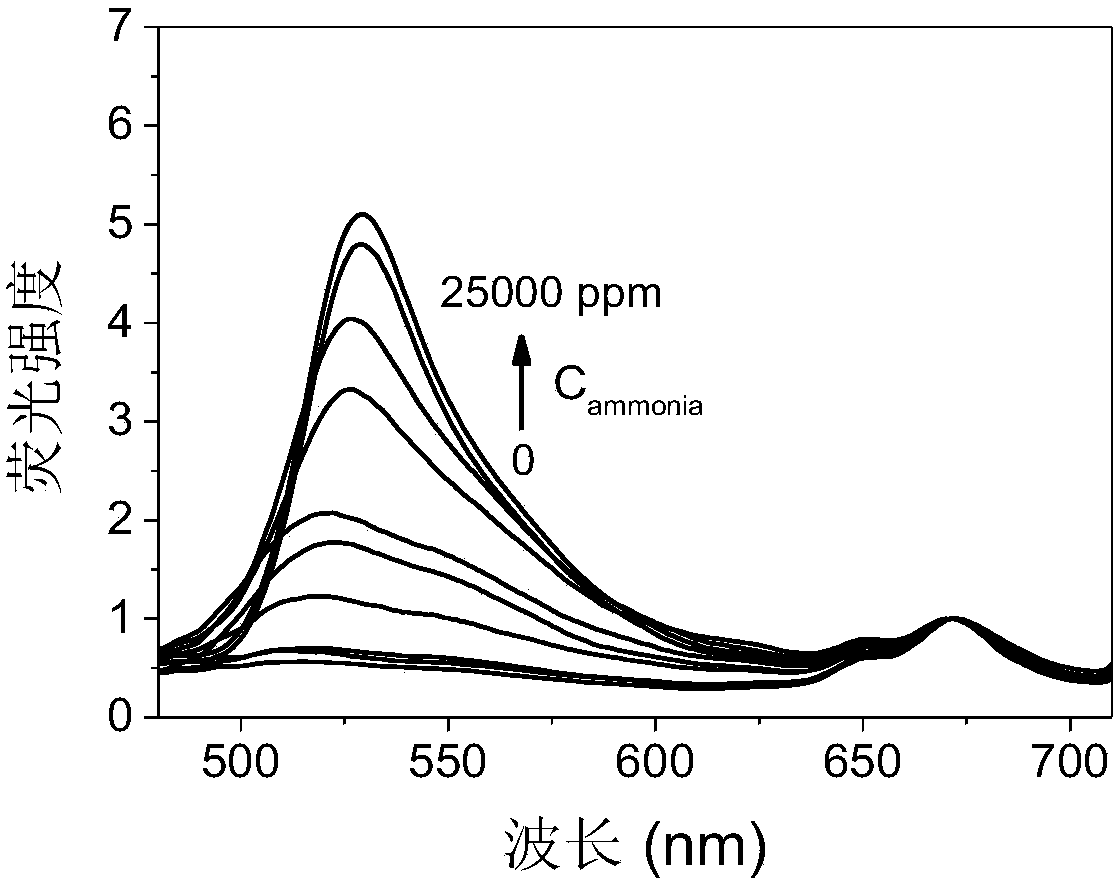
[0086] The fluorescent group of fluorescein isothiocyanate and the fluorescent group of protoporphyrin in the double-emission cellulose-based fluorescent material are bonded on the same cellulose, wherein the fluorescent group of fluorescein isothiocyanate is bound to the biogenic amine Responsive, protoporphyrin fluorophore is not re...
Embodiment 2
[0089] Preparation of dual-emission cellulose-based fluorescent materials by solid blending method
[0090] Weigh 0.9478g cellulose acetate and 0.0389g fluorescein isothiocyanate to carry out esterification reaction under the catalytic condition of dibutyltin dilaurate (esterification reaction temperature is 100 ℃; esterification reaction time is 4h), will The obtained product is precipitated, washed and dried for later use.
[0091] Weigh 0.9478g cellulose acetate and 0.0563g protoporphyrin to carry out esterification reaction under the catalysis of carbonyldiimidazole (esterification reaction temperature is 80°C; esterification reaction time is 20h), the obtained product is precipitated, washed and dried for backup use.
[0092] Mix the cellulose and its derivatives bonded to the isothiocyanate fluorescein fluorescent group and the cellulose and its derivatives bonded to the protoporphyrin fluorescent group with a mass ratio of 1:5 , and ground in a mortar to prepare a dou...
Embodiment 3
[0098] Preparation of Dual Emission Cellulose-Based Fluorescent Materials by Solution Blending
[0099] Weigh 0.9478g of cellulose nitrate and 0.0389g of fluorescein isocyanate to carry out esterification reaction under the catalytic conditions of dibutyltin dilaurate (esterification reaction temperature is 100°C; esterification reaction time is 4h), and the resulting product is precipitated , Wash and dry for later use.
[0100]Weigh 0.9478g of methylcellulose and 0.0563g of protoporphyrin to carry out esterification reaction under the catalysis of carbonyldiimidazole (esterification reaction temperature is 80°C; esterification reaction time is 20h), and the resulting product is precipitated, washed and dried Backup.
[0101] Dissolve the cellulose derivatives bonded to the isocyanate fluorescein fluorescent group and the cellulose derivatives bonded to the protoporphyrin fluorescent group that were synthesized respectively in N,N-dimethylformamide, and then the above two T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com