Crackable chain small in chemical steric hindrance and preparation method and application of crackable chain
A steric hindrance and chemical technology, applied in the field of prodrugs and fluorescent probe materials, can solve the problems of serious influence of esterification reaction, limited design and synthesis of prodrug models and fluorescent probe materials, difficult connection of responsive groups, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Synthesis of Example 1 Compound 2
[0081] 1.0 g 5.95 mmol of compound 1 (2,6-bis(hydroxymethyl)-4-methylphenol) and 5.0 g 17.86 mmol of 2-iodoylbenzoic acid were placed in a round bottom flask, its compounds 1 and 2 -The molar ratio of iodoyl benzoic acid is 1:3, add 20 mL of ethyl acetate, heat under reflux at 70°C for 3 h, cool to room temperature, filter, and wash the filter cake with ethyl acetate for 2 to 3 times, remove the organic solvent by rotary evaporation , carry out column chromatography with an eluent whose volume ratio is petroleum ether:ethyl acetate=10:1 to obtain compound 2 as a white solid;
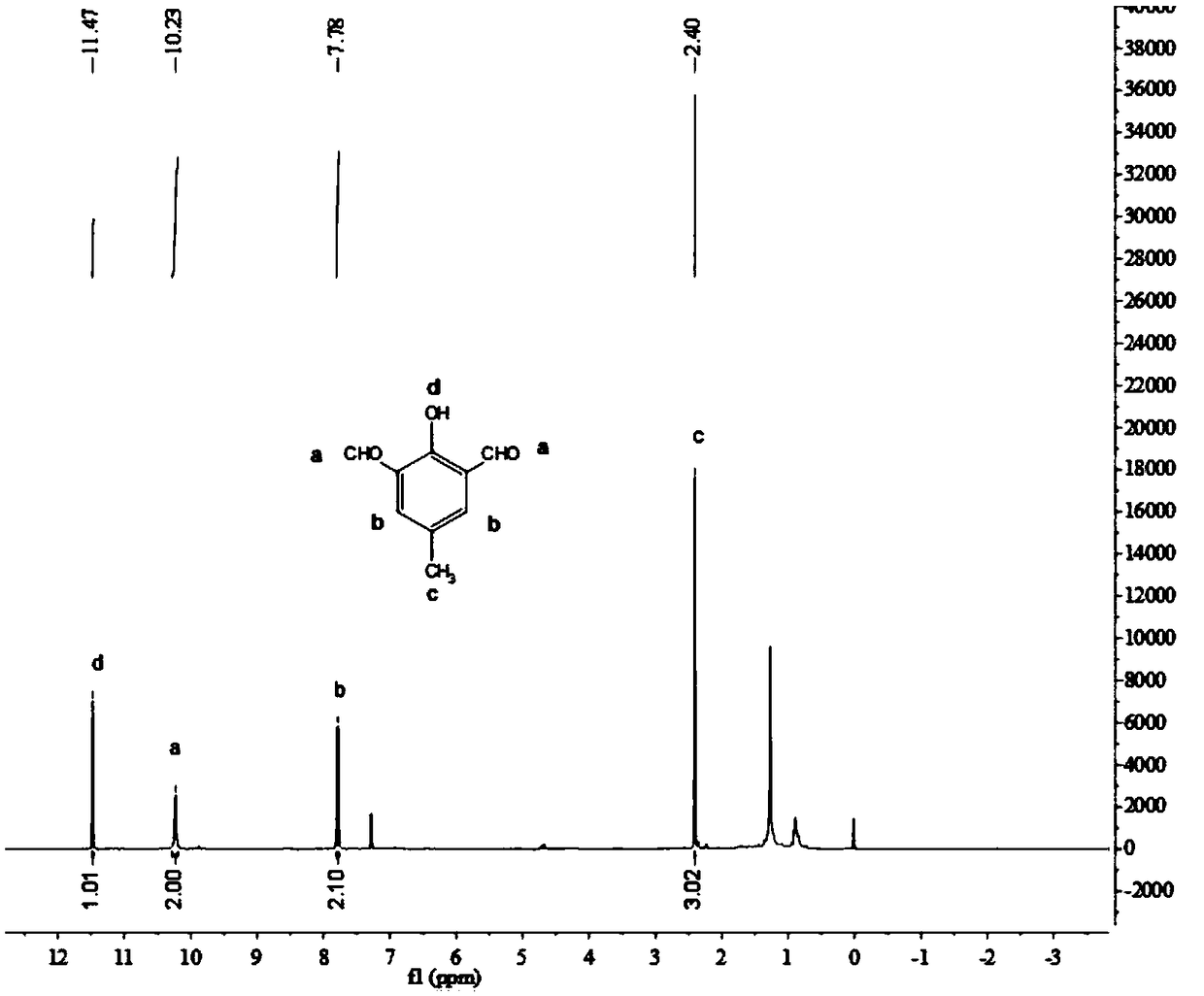
[0082] The obtained compound 2 is 2-hydroxy-5-methyl isophthalaldehyde, and the structural formula of its compound 2 is The yield was 76.3%;
[0083] Compound 2 was characterized by H NMR spectroscopy, and the results were as follows figure 1 As shown, the characterization data is as follows: 1 HNMR (400MHz, CDCl 3 ,ppm)δ11.47(s,1H), 10.23(s,2H), 7.78(s,2H...
Embodiment 2
[0084] Example 2 Synthesis of Compound 3
[0085] 745 mg 4.543 mmol of compound 2 obtained in Example 1 and 6.3 g of 18.1 mmol of ethoxyformylmethylene triphenylphosphine were placed in a round bottom flask, and its compound 2 was mixed with ethoxyformylmethylene triphenylphosphine. The molar ratio of phosphine was 1:4, 30 mL of dichloromethane was added, and the reaction was stirred at room temperature for 6 h; after the reaction was completed, the solvent was removed by rotary evaporation, and the eluent was carried out with a volume ratio of petroleum ether:ethyl acetate=1.5:1. Column chromatography gave compound 3 as a white solid;
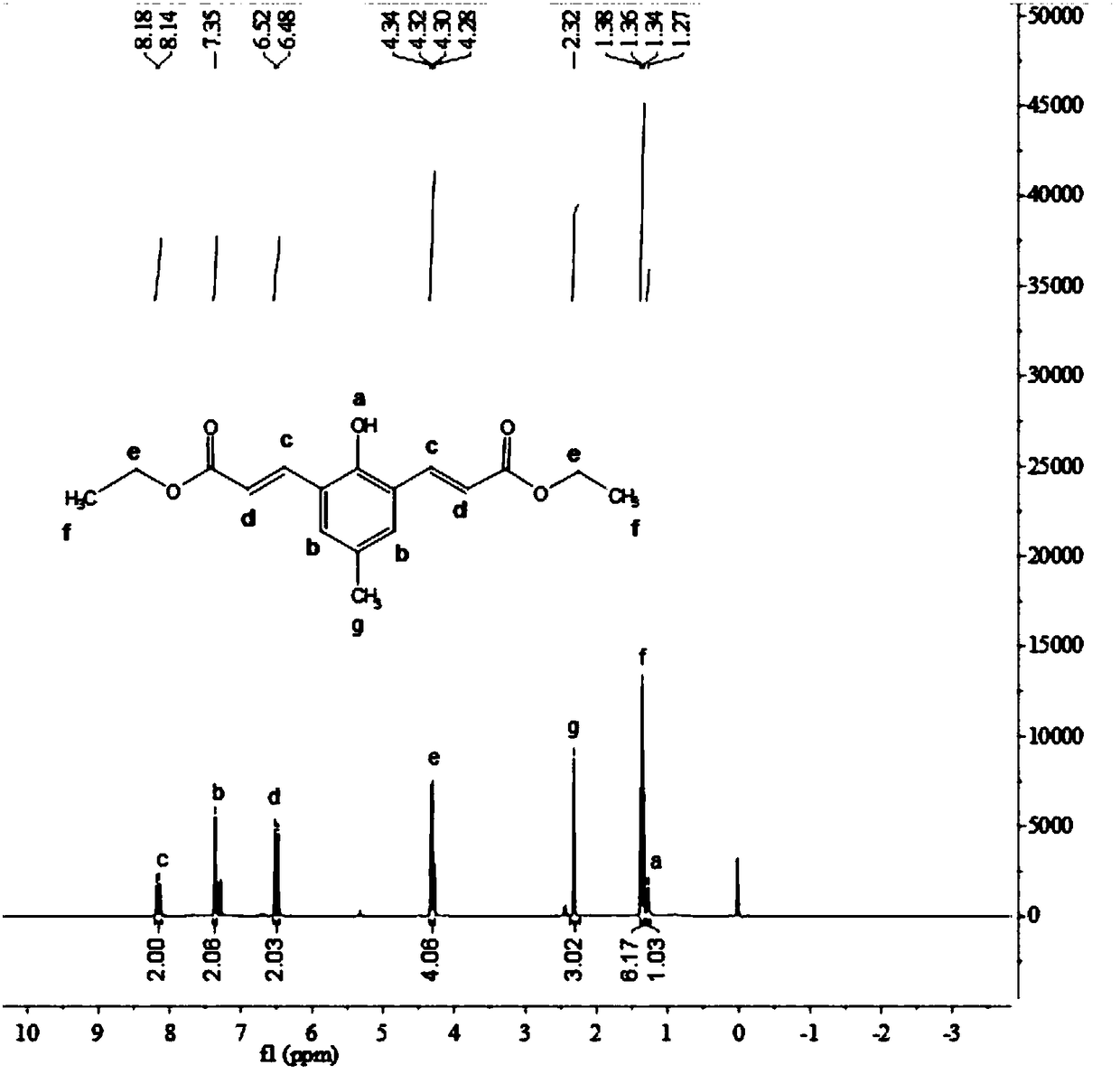
[0086] The structural formula of the obtained compound 3 is The yield was 86.4%.
[0087] Compound 3 was characterized by H NMR spectroscopy, and the results were as follows figure 2 As shown, the characterization data is as follows: 1 HNMR (400MHz, CDCl 3 ,ppm)δ8.10~8.20(d,2H), 7.35(s,2H), 6.45~6.55(d,2H), 4.25~4.40(m,4H), 2.32(s,1H), ...
Embodiment 3
[0088] Example 3 Synthesis of Severable Chains
[0089] 800 mg 2.63 mmol of compound 3 obtained in Example 2 was placed in a round-bottomed flask, 30 mL of tetrahydrofuran was added to dissolve it, cooled to -5°C under nitrogen protection, and 10.5 mL of 10.5 mmol of diisobutylaluminum hydride ( 1M n-hexane solution) was slowly added dropwise to the reaction flask, the molar ratio of compound 3 and diisobutylaluminum hydride was 1:4, and the reaction was carried out at -5 °C for 1 h; after the reaction was completed, 20 mL of water was slowly added dropwise. Quenched, extracted three times with ethyl acetate, filtered when a white solid was precipitated, and dried the filtrate with anhydrous sodium sulfate; finally, eluted with a volume ratio of petroleum ether:ethyl acetate=1:2 The agent is subjected to column chromatography to obtain a yellow solid product;
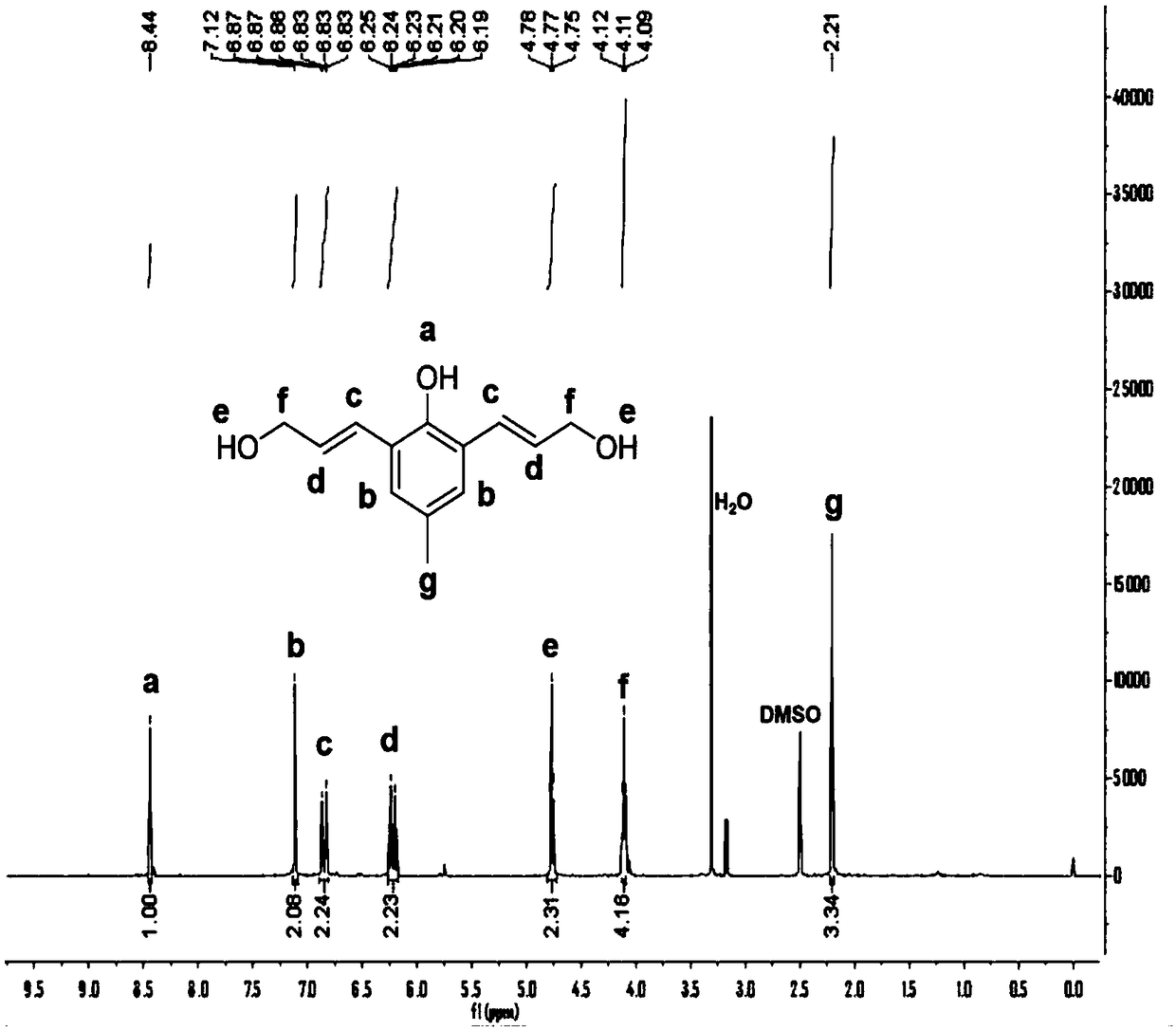
[0090] The yellow solid product is a cleavable chain, and its molecular formula is C 13 H 16 O 3 , the structural...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com