A kind of bcma chimeric antigen receptor based on single domain antibody and its application
A technology of chimeric antigen receptors and carriers, which is applied in the fields of receptors/cell surface antigens/cell surface determinants, antibody medical components, antibody mimics/scaffolds, etc. It can solve the problems of patients with less than 20% survival rate and repeated recurrences. problem, to achieve the effect of strong killing effect, strong lethality and good healing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1B
[0092] Example 1 BCMA single domain antibody VHH gene acquisition
[0093] 1) Construction of BCMA single domain antibody library
[0094] Adult healthy alpacas were immunized with multiple subcutaneous injections on the back of the neck with BCMA antigen purchased from Beijing Yiqiao Shenzhou. During immunization, add antigen and an equal volume of Freund's adjuvant, immunize in 4-6 times, follow up and observe the absorption of mass at the injection site to confirm the correct immunization. The interval between immunizations is 7-15 days. After the 4th immunization, blood is collected to determine the antigen immune titer. When the titer reaches 10,000 times or more (ELISA method), about 100ml of blood is collected, lymphocytes are separated, RNA is extracted, and reversed. It was recorded as cDNA, and the variable region fragment VHH of alpaca heavy chain antibody was amplified by two PCRs. The VHH fragment is constructed into a phage display library, and the product of t...
Embodiment 2
[0099] Example 2 Construction of Chimeric Antigen Receptor Gene Vector
[0100] Two sections of genes were synthesized by Zhongmei Taihe Company, one section of nucleotide sequence SEQ ID NO: 15 contained in BCMA sdAb I prepared in Example 1; one section is the designed 2nd generation CAR structural gene (CD8a hinge region, transmembrane domain+ 4-1BB co-stimulatory domain + CD3ζ intracellular signaling domain, the nucleotide sequence encoding the 2nd generation CAR structural gene comprising these domains is shown in SEQ ID NO: 16). After obtaining the synthetic gene, carry out molecular cloning, carry out the construction of BCMA CAR, obtain the PCR product of SEQ ID NO:15 and SEQ ID NO:16 by PCR, carry out overlapping PCR, obtain the BCMACAR gene (the nucleus of which two fragments are connected together) The nucleotide sequence is shown in SEQ ID NO: 12); the lentiviral Pre vector and the BCMA CAR gene were digested, ligated, transformed, cloned, plasmid extracted, and seq...
Embodiment 3B
[0105] Example 3 Preparation of BCMA Chimeric Antigen Receptor Lentivirus
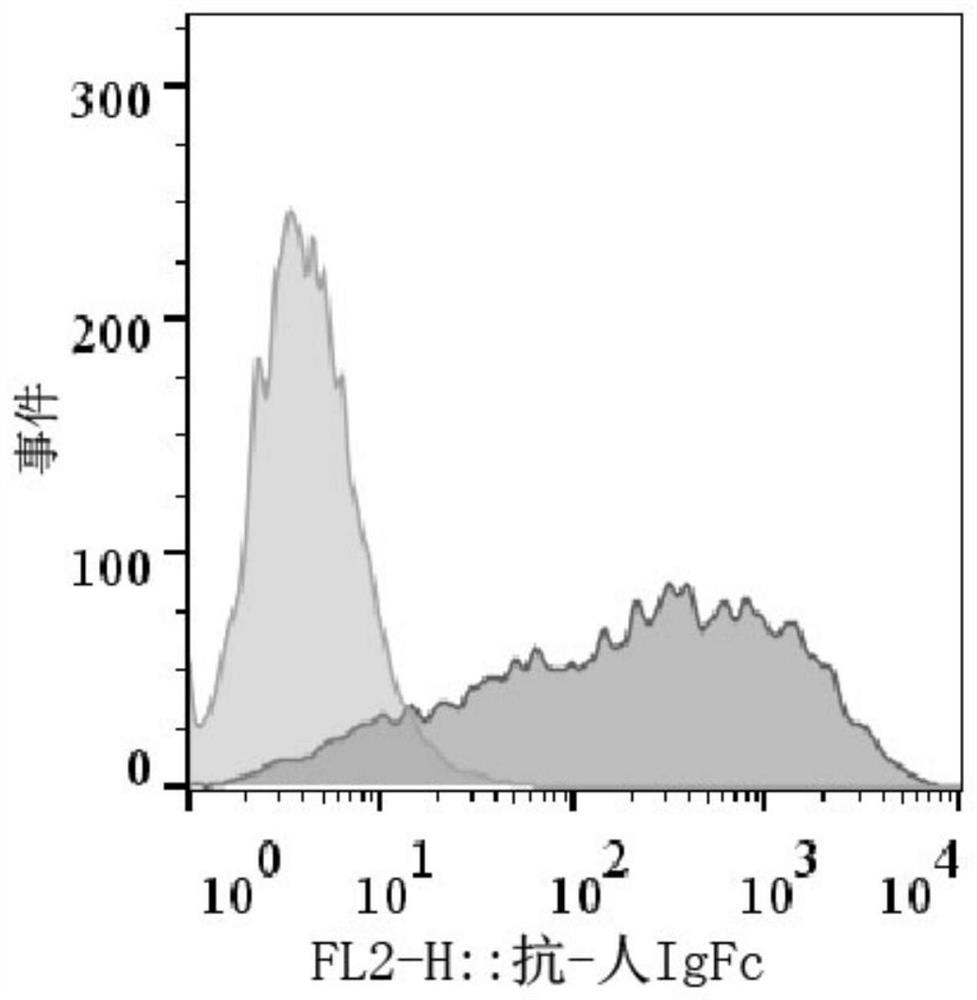
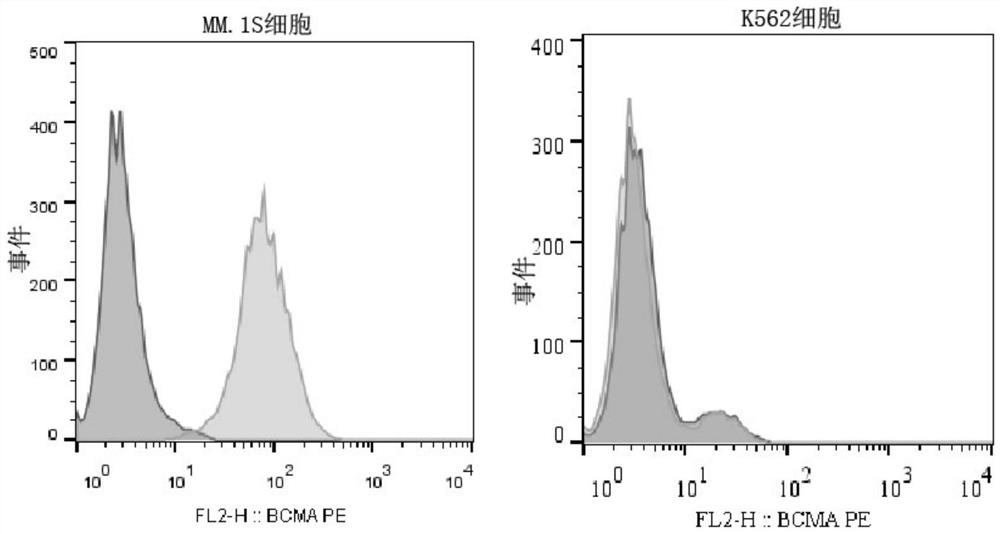
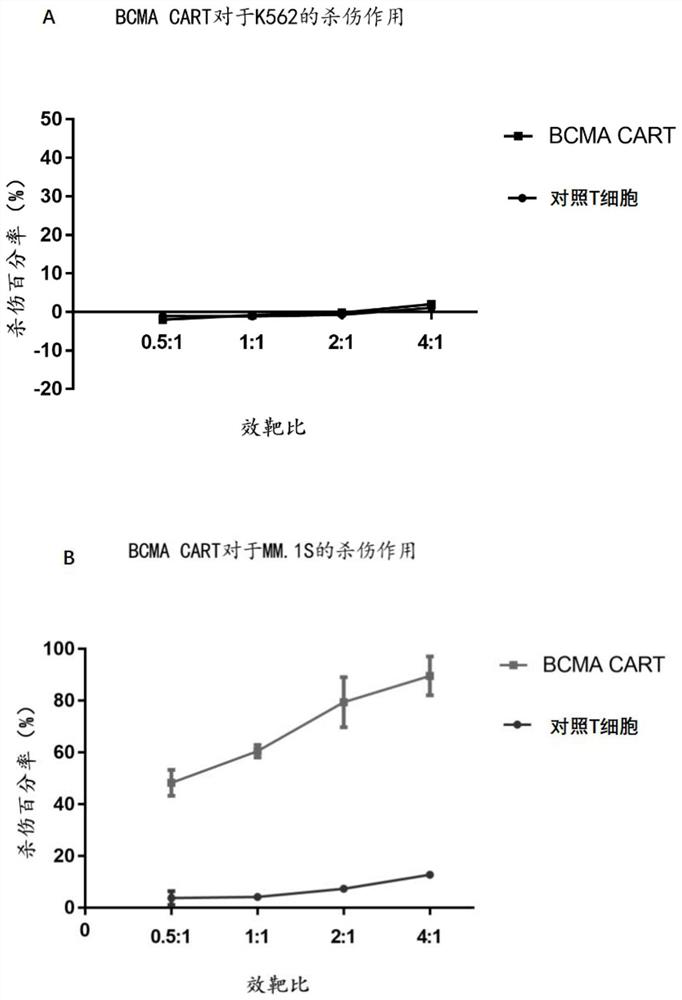
[0106] The day before packaging the virus, trypsinize 293T cells (purchased from ATCC), 1×10 7 Cells / dish were planted in 10cm culture dish. When transfecting cells, except for the Pre-Lenti-EF1-BCMA plasmid prepared in Example 2, each plasmid needs to be co-transfected with packaging plasmids psPAX2 and pMD2.0G. Among them, 5 μg of Pre-Lenti-EF1-BCMA, 3.75 μg of psPAX2 plasmid and 1.25 μg of pMD2.0G plasmid were used. For transfection, add the mixture of the above three plasmids to 500 μl MEM medium, add 25 μl Lipofectamine (liposome) 2000 reagent to 500 μl MEM medium in another microcentrifuge tube, and then add the diluted transfection reagent one by one Add dropwise on top of the diluted plasmid, mix well, centrifuge, and stand at room temperature for 20 minutes. Finally, add the mixture of plasmid and transfection reagent into a 10cm culture dish, shake gently 10 times, mix well, and put it in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com