A dual-target tumor vaccine and its preparation method and application
A tumor vaccine and dual-target technology, applied in the field of biomedicine, can solve the problems of weak immunogenicity and small polypeptide molecules, and achieve the effects of promoting apoptosis, improving effectiveness and safety, and promoting cascade system.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
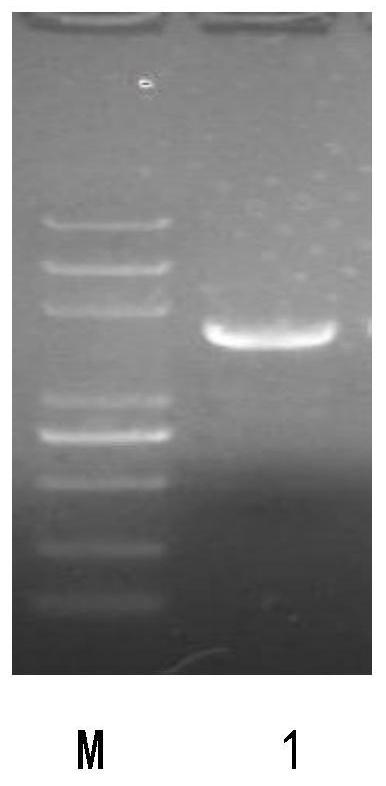
[0041] Preparation of Example 1 GPR124 Gene Recombination Eukaryotic Expression Plasmid pcDNA3.5 / GPR124
[0042] (1) Cloning of the full-length coding gene of GPR124
[0043] According to the GPR124 gene sequence with the GenBank accession number NM_054044.2, PCR primers were designed and synthesized to amplify the full-length coding gene of GPR124; PCR was performed using the human placenta cDNA library as a template. Denaturation at ℃ for 30 seconds, annealing at 56℃ for 30 seconds, extension at 72℃ for 30 seconds, a total of 30 cycles, and finally extension at 72℃ for 5 minutes. Ligated with the vector pGEM-T, the ligated product was transformed into Escherichia coli JM109 competent cells, and the medium containing Amp, IPTG, X-GAL was used for blue and white spot screening, the white spot was picked and cultured, the plasmid was extracted, and the gene sequence was determined by Shanghai Shenggong Company , named the positive clone plasmid with correct sequence as pGEM-T / ...
Embodiment 2
[0048] Preparation of the fusion peptide of embodiment 2 membrane-penetrating peptide and AVPI peptide
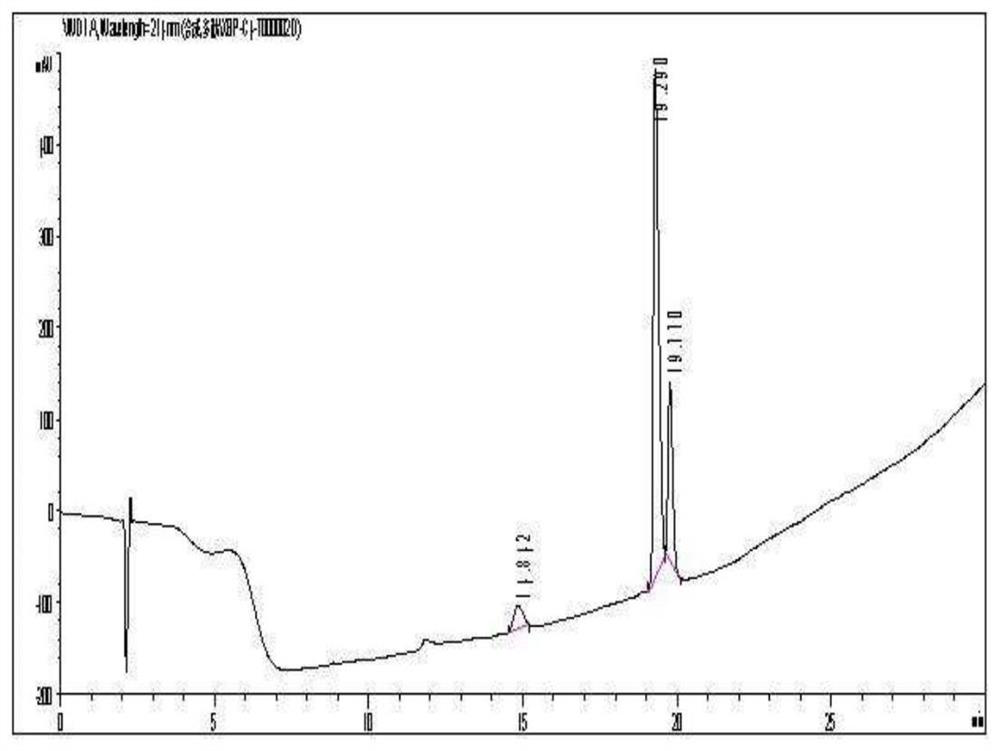
[0049] The fusion peptide is formed by directly linking the carboxyl terminus of the penetrating peptide and the amino terminus of the AVPI peptide, and its amino acid sequence is shown in SEQID No.1.
[0050] The fusion peptide was solid-phase synthesized on an AB-431A peptide synthesizer, using a standard fluorenylmethoxycarbonyl (Fmoc) protocol, with 0.25 mmol of p-hydroxymethylphenoxymethyl polystyrene (HMP) resin as the starting resin, According to the amino acid sequence of SEQ ID No.1, the peptide chain is extended from the carboxyl terminal to the amino terminal one by one. After the synthesis of the peptide chain is completed, the resin containing the peptide chain is transferred to a cutting solution (0.25 mL of ethylenediamine tartrate, trifluoroacetic acid 9.5mL and 0.25mL of deionized water), stir the reaction at room temperature to crack the peptide chain from...
Embodiment 3
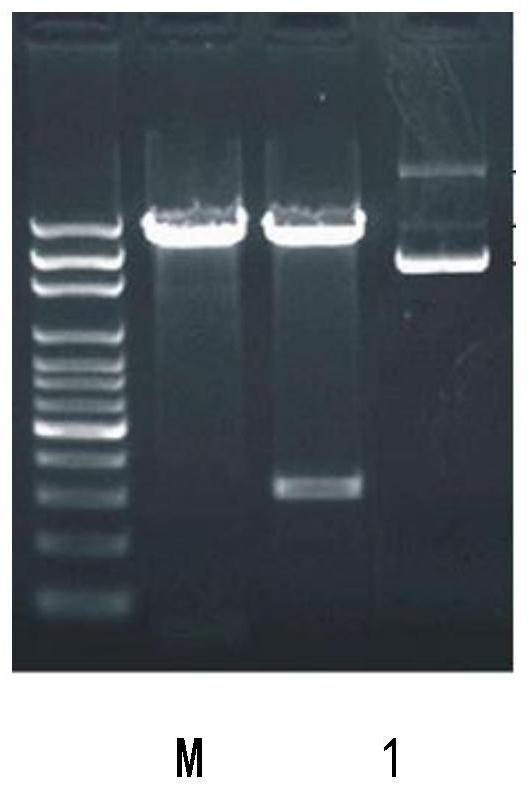
[0053] The preparation of embodiment 3 tumor vaccines
[0054] At room temperature under the condition of vortexing, add 1 volume part of fusion peptide with a concentration of 1 mg / ml dropwise to 1 volume part of an aqueous solution containing pcDNA3.5 / GPR124 at a concentration of 1 mg / ml and PBS with a concentration of 1 mg / ml Peptide solution, after the dropwise addition, continued to swirl for 60 minutes, and then stood still for 60 minutes to obtain the tumor vaccine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com