11/2 water citicoline sodium compound and pharmaceutical composition preparation
A technology of citicoline sodium and its compound, which is applied in the field of chemical engineering and pharmaceutical crystallization, can solve the problems of high moisture absorption, poor stability, and backward preparation technology, and achieve the effects of not easy to absorb moisture, stable properties, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
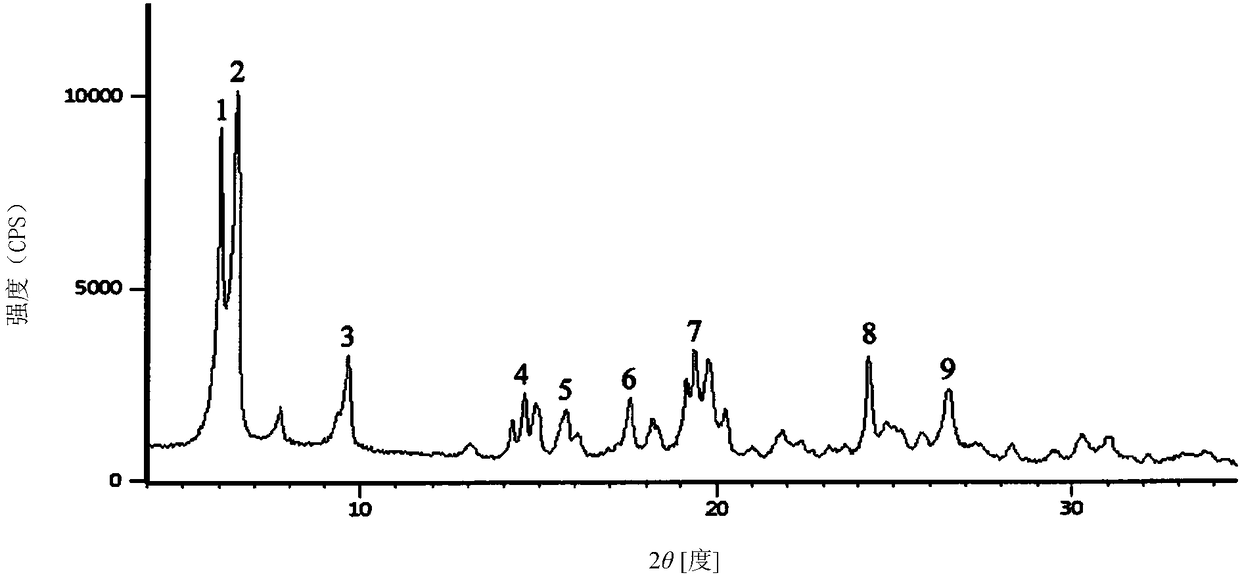
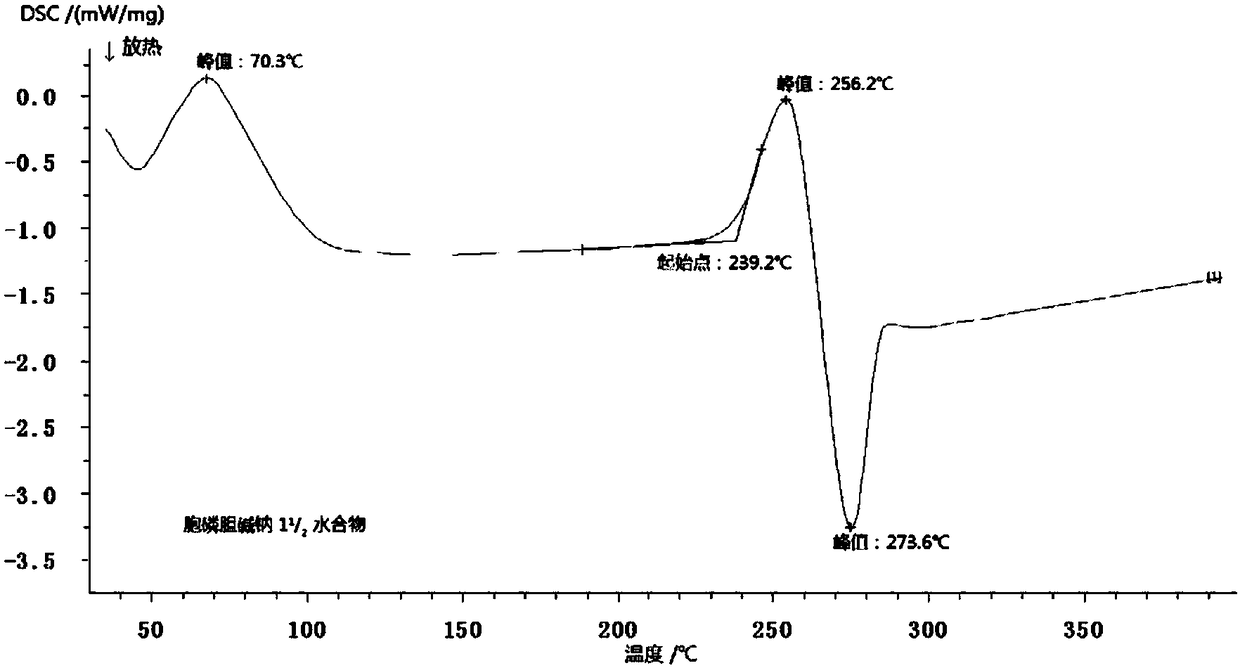
[0029] Example 1 1 1 / 2 Preparation of Cytocholine Sodium Compound
[0030] Preparation process: Add 200 mL of purified water into the reactor, weigh 100.2 g of crude citicoline sodium into the reactor, and stir until dissolved at room temperature. Add 0.3g of activated carbon to it, stir and adsorb for 30min, filter and decarbonize, adjust the pH to 1.5 with 1mol / L hydrochloric acid, stir well, control the temperature at 15-20°C, slowly add 100mL of ethanol to the above solution, wait until the ethanol is added dropwise After completion, add 0.5g of seed crystals, control the temperature at 5-8°C, let stand for crystallization for 1.5h, and filter; wash the filtrate with 80mL×2 ethanol, and vacuum-dry at 60°C for 2.5h to obtain 1 1 / 2 Hydrocytoline Sodium Compound 99.6g.
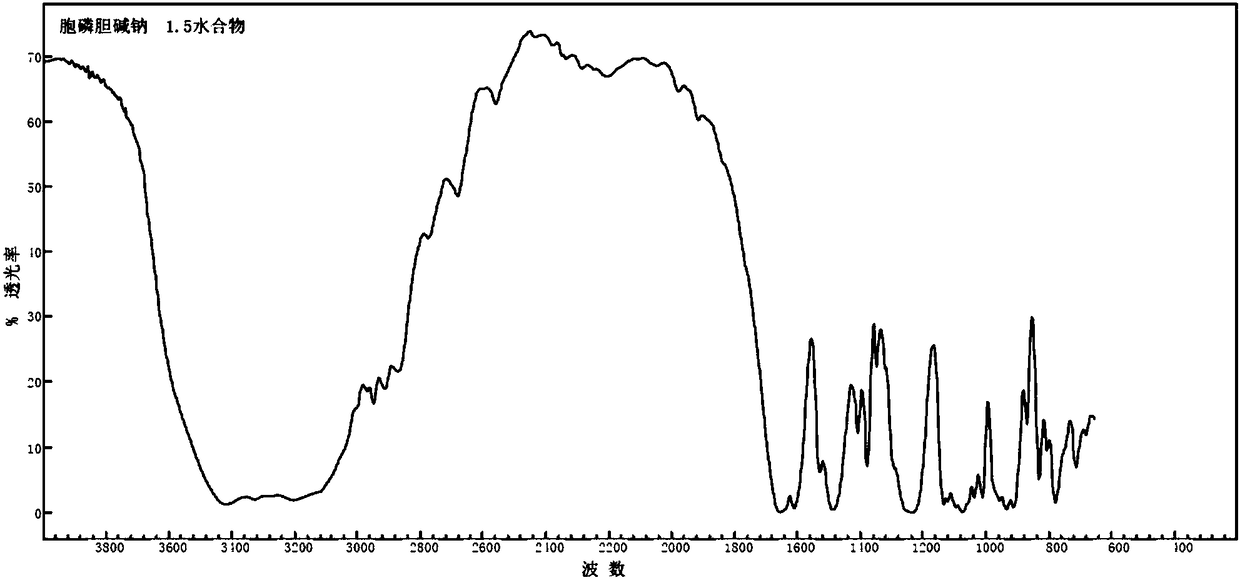
[0031] Infrared spectrum at wavenumber 3414.8cm -1 , 3204.3cm -1 , 1654.2cm -1 , 1612.0cm -1 , 1487.3cm -1 , 1380.0cm -1 , 1234.2cm -1 , 1135.3cm -1 , 1072.2cm -1 , 1014.3cm -1 ,833.2cm -1 ...
Embodiment 2
[0036] Example 2 1 1 / 2 Preparation of Cytocholine Sodium Compound
[0037] Preparation process: Add 250 mL of purified water into the reactor, weigh 100.3 g of crude citicoline sodium into the reactor, and stir until dissolved at room temperature. Add 0.3g of activated carbon to it, stir and adsorb for 30min, filter and decarbonize, adjust the pH to 2.0 with 1mol / L hydrochloric acid, stir well, control the temperature at 20-25°C, slowly add 100mL of ethanol to the above solution, wait until the ethanol is added dropwise After completion, add 0.6g of seed crystals, control the temperature at 8-10°C, let stand for crystallization for 1h, and filter; wash the filtrate with 80mL×2 ethanol, and vacuum-dry at 70°C for 2h to obtain 1 1 / 2 Cytophosphacholine Sodium Compound 99.5g.
[0038] The infrared spectrum has a wavenumber of 3414.5cm -1 , 3204.1cm -1 , 1654.3cm -1 , 1612.2cm -1 , 1487.4cm -1 , 1380.3cm -1 , 1234.5cm -1 , 1135.4cm -1 , 1072.4cm -1 , 1014.6cm -1 ,...
Embodiment 3
[0043] Example 3 1 1 / 2 Preparation of hydrocytoline sodium pharmaceutical composition preparation
[0044] Take the citicoline sodium compound prepared in Example 2, and use this raw material to prepare citicoline sodium injection, the specification is 2ml: 0.1g.
[0045] prescription:
[0046]
[0047] Preparation Process:
[0048] 1. Liquid preparation:
[0049] (1) Add 80% water for injection of the total amount in the liquid distribution tank;
[0050] (2) Add 1 of the prescription amount under the stirring state 1 / 2 Cytocholine Sodium, stir to dissolve completely;
[0051] (3) adjust the pH value to 7.0~7.5 with 0.2mol / L sodium hydroxide solution;
[0052] (4) Add water for injection to the full amount;
[0053] (5) Add activated carbon with a total volume of 0.05% (w / v), stir and adsorb for 15 minutes;
[0054] (6) Coarse filtration and decarburization of the solution: filter through a 0.45 μm filter;
[0055] 2. Intermediate detection: detect the pH va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com