Impurity analysis method for multiple vitamin preparations
A vitamin preparation and impurity analysis technology, which is applied to the analysis of materials, material separation, instruments, etc., can solve the problems of low ultraviolet absorption of impurities, large differences in vitamin content, and influence on accuracy, so as to improve separation and sensitivity, and ultraviolet absorption The effect of capacity enhancement and retention time increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
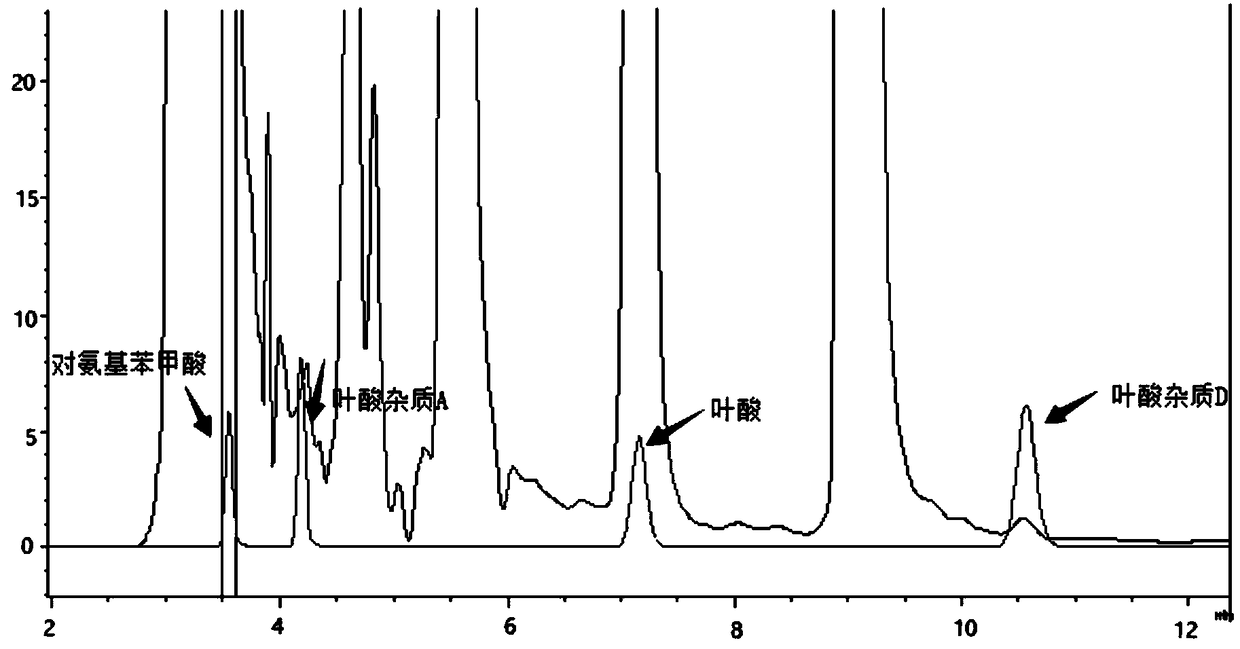
[0079] Take the multivitamin preparation, dissolve it in water and dilute it to about 120 μg folic acid per 1 ml solution, as the test solution;
[0080] Take folic acid impurity A reference substance, folic acid impurity D reference substance, p-aminobenzoic acid reference substance and 3-aminopropanol reference substance, add 1ml 28mg / L sodium carbonate solution to dissolve, dilute with 10% methanol solution to every 1ml solution Containing 2.4 μg of folic acid impurity A, 2.4 μg of folic acid impurity D, 1.2 μg of p-aminobenzoic acid, and 3 μg of 3-aminopropanol, as the reference solution;
[0081] Take 25 mg of o-phthalaldehyde and 55 mg of 2-mercaptoethanol, add 0.5 ml of methanol to dissolve, and then add 5 ml of 0.4 mol / L boric acid buffer solution (adjust the pH value to 4.5 with phosphoric acid) to obtain a derivatization reagent;
[0082] Take 10 μL each of the above-mentioned test solution and reference solution, add 30 μL of derivatization reagents to carry out onl...
Embodiment 2
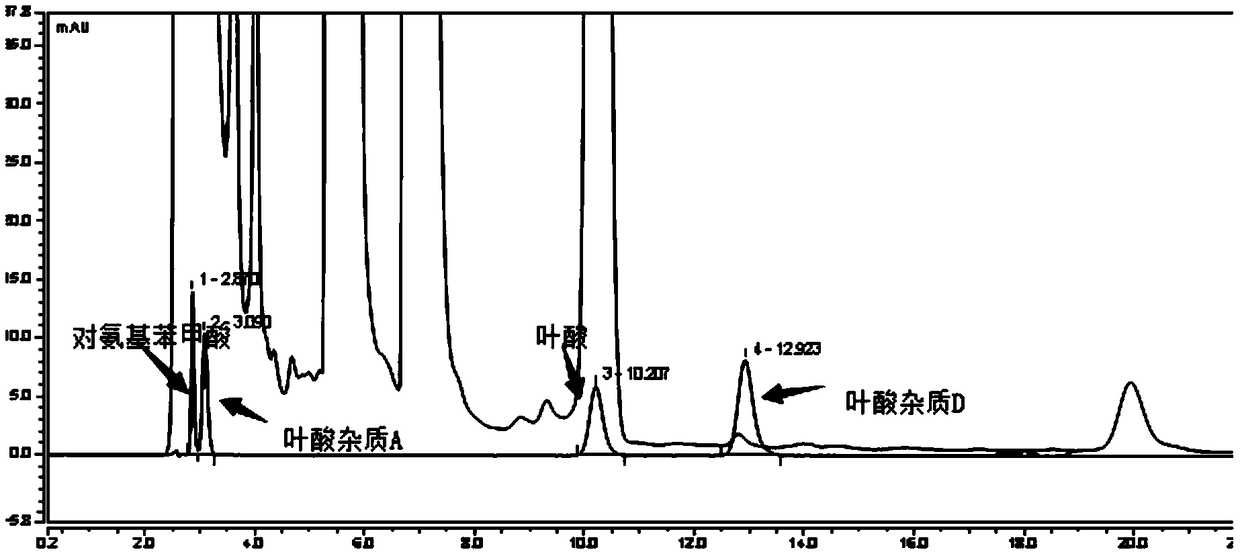
[0095] Take the multivitamin preparation, dissolve it in water and dilute it to about 120 μg folic acid per 1 ml solution, as the test solution;
[0096] Take folic acid impurity A reference substance, folic acid impurity D reference substance, p-aminobenzoic acid reference substance and 3-aminopropanol reference substance, add 1ml 28mg / L sodium carbonate solution to dissolve, dilute with 10% methanol solution to every 1ml solution Containing 2.4 μg of folic acid impurity A, 2.4 μg of folic acid impurity D, 1.2 μg of p-aminobenzoic acid, and 3 μg of 3-aminopropanol, as the reference solution;
[0097] Take 25 mg of o-phthalaldehyde and 55 mg of 2-mercaptoethanol, add 0.5 ml of methanol to dissolve, and then add 5 ml of 0.4 mol / L boric acid buffer solution (adjust the pH value to 4.5 with phosphoric acid) to obtain a derivatization reagent;
[0098] Take 10 μL each of the above-mentioned test solution and reference solution, respectively add 50 μL of derivatization reagent to c...
Embodiment 3
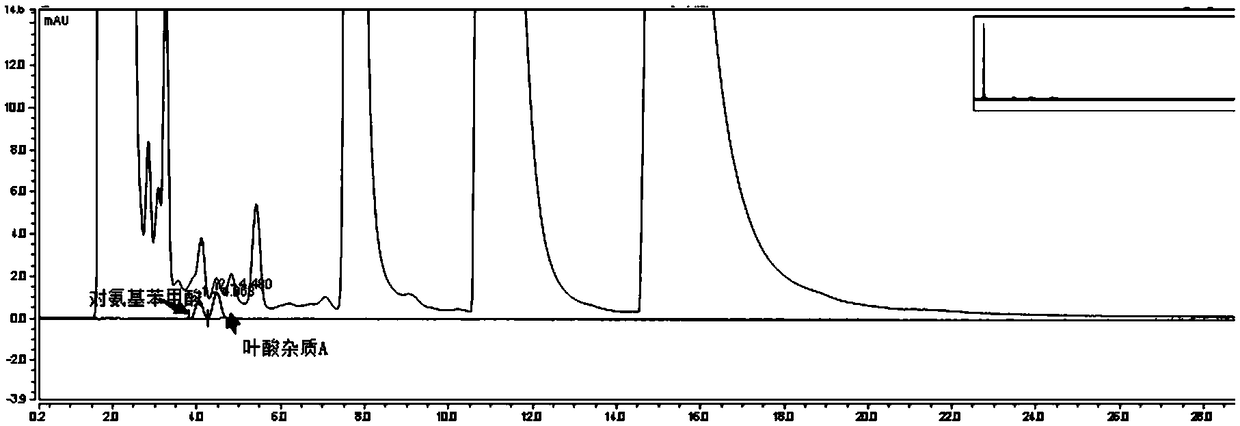
[0102] Take the multivitamin preparation, dissolve it in water and dilute it to about 120 μg folic acid per 1 ml solution, as the test solution;
[0103] Take folic acid impurity A reference substance, folic acid impurity D reference substance, p-aminobenzoic acid reference substance and 3-aminopropanol reference substance, add 1ml 28mg / L sodium carbonate solution to dissolve, dilute with 10% methanol solution to every 1ml solution Containing 2.4 μg of folic acid impurity A, 2.4 μg of folic acid impurity D, 1.2 μg of p-aminobenzoic acid, and 3 μg of 3-aminopropanol, as the reference solution;
[0104] Take 25 mg of o-phthalaldehyde and 55 mg of 2-mercaptoethanol, add 0.5 ml of methanol to dissolve, and then add 5 ml of 0.4 mol / L boric acid buffer solution (adjust the pH value to 4.5 with phosphoric acid) to obtain a derivatization reagent;
[0105] Take 10 μL each of the above-mentioned test solution and reference solution, add 30 μL of derivatization reagent for derivatizatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com