A montmorillonite-based composite solid electrolyte and a solid-state lithium battery
A solid electrolyte, montmorillonite-based technology, applied in solid electrolytes, non-aqueous electrolytes, secondary batteries, etc., to achieve the effects of wide ion migration number, excellent cycle and rate performance, and high room temperature ion conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
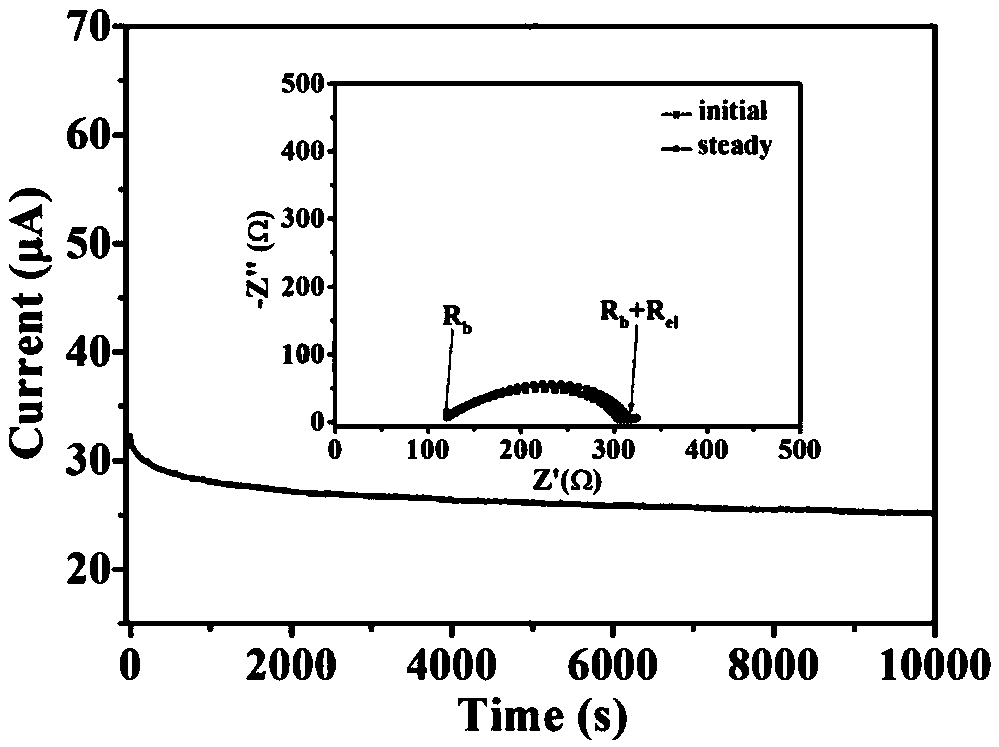
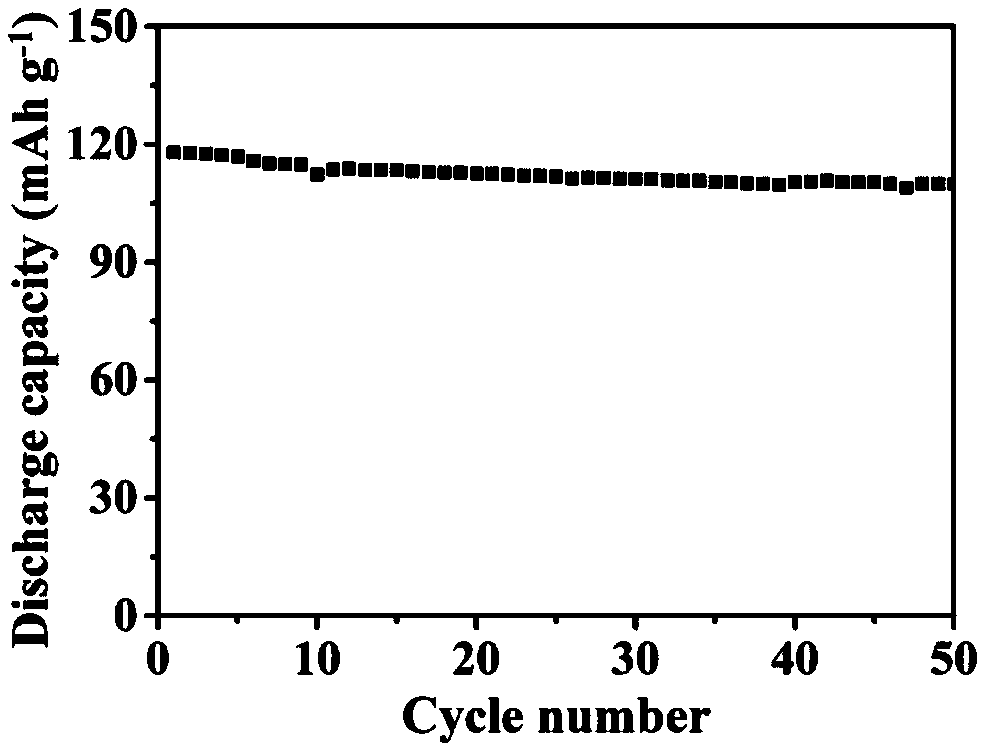
[0028]First, fully dissolve hectorite, polyacrylonitrile and lithium salt LiTFSI with a mass ratio of 50:12:25 in acetonitrile in an argon glove box, stir for 18 hours to obtain a uniform viscous slurry, and then cast the slurry Put it into a polytetrafluoroethylene mold, dry it at room temperature for 20 hours, and dry it under vacuum at 45°C for 60 hours to remove the residual solvent to obtain a polymer / hectorite solid mixture. Afterwards, FEC and PTFE binders with mass fractions of 10% and 3% were respectively added to the above mixture, and after being fully mixed, they were pressed at 13MPa for 5min at a temperature of 30°C to obtain a montmorillonite-based composite solid electrolyte with a thickness of 70μm. . The surface morphology of the composite solid electrolyte was observed by scanning electron microscopy (SEM). The surface of the electrolyte membrane was smooth and even, and the inorganic particles were evenly distributed. The room temperature ionic conductivit...
Embodiment 2
[0030] First, hectorite, polypropylene oxide, and lithium salt LiClO with a mass ratio of 45:10:30 were mixed in an argon glove box. 4 Dissolved in acetonitrile, stirred for 36 hours to obtain a uniform viscous slurry, then cast the slurry in a polytetrafluoroethylene mold, dried at room temperature for 40 hours, and dried in vacuum at 50°C for 65 hours to remove residual solvent to obtain a polymer / hectorite solid mixture. Afterwards, TB and PTFE binders with mass fractions of 8% and 2% were respectively added to the above mixture, and after thorough mixing, they were pressed at 12MPa for 25min at 40°C to obtain a composite solid electrolyte membrane with a thickness of 60μm. The surface morphology of the composite solid electrolyte was observed by scanning electron microscopy (SEM). The surface of the electrolyte membrane was smooth and even, and the inorganic particles were evenly distributed. The ionic conductivity of the composite electrolyte at room temperature is 2.8×1...
Embodiment 3
[0032] First, hectorite, polyethylene oxide and lithium salt LiBF with a mass ratio of 50:8:35 were mixed in an argon glove box. 4 Fully dissolved in acetonitrile, stirred for 30 hours to obtain a uniform viscous slurry, then cast the slurry in a polytetrafluoroethylene mold, dried at room temperature for 35 hours, and dried under vacuum at 60°C for 50 hours to remove residual solvents to obtain polymer / hectorite solid mixture. Afterwards, ETFEC and PTFE binders with a mass fraction of 2% and 5% respectively were added to the above mixture, and after thorough mixing, the composite solid electrolyte membrane with a thickness of 65 μm was obtained by pressing at 10 MPa for 20 minutes at a temperature of 45°C. The surface morphology of the electrolyte membrane was observed by scanning electron microscopy (SEM). The surface of the electrolyte membrane was smooth and even, and the particles were uniformly dispersed. The room temperature ionic conductivity of the composite electrol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com