Preparation method of 4-hydroxyl-3-substuted-thiazolane-2-ketone compound
A technology of ketone compounds and thiazolidine, which is applied in the field of medicine, can solve the problems of low product yield and achieve the effects of low cost, mild reaction conditions and high-efficiency synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
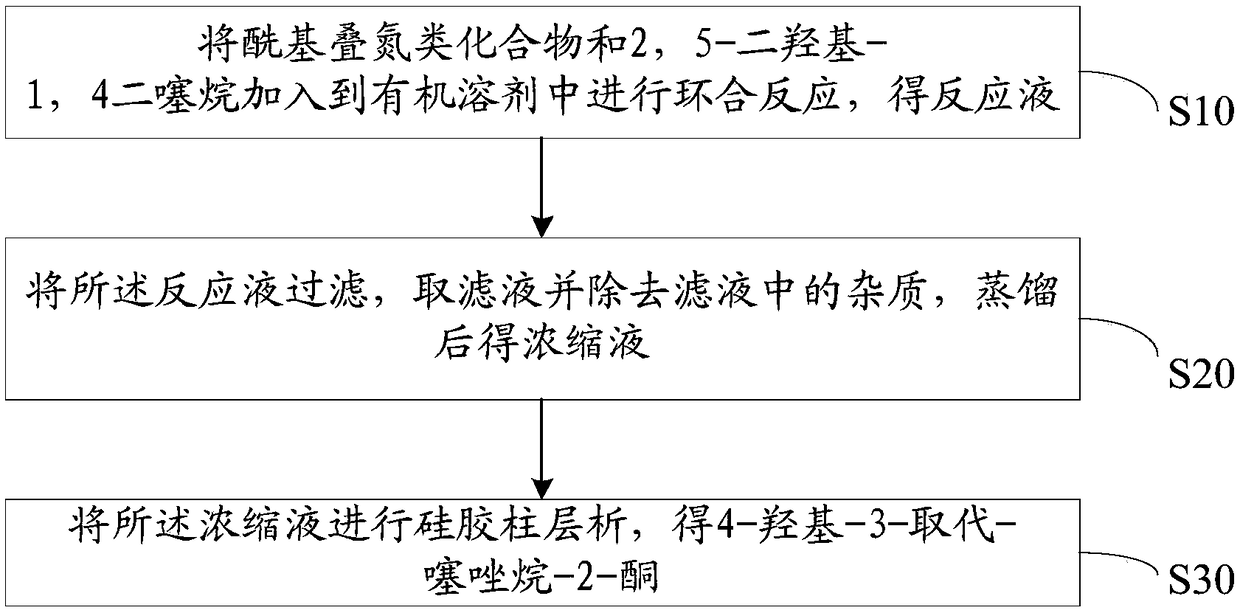
[0029] In view of this, the present invention proposes a preparation method of 4-hydroxy-3-substituted-thiazolidin-2-one compounds. combine figure 1 The schematic flow diagram of an embodiment of the preparation method of the shown 4-hydroxyl-3-substituted-thiazolidin-2-one compound, the preparation of the 4-hydroxyl-3-substituted-thiazolidin-2-one compound The method includes the following steps:
[0030] Step S10, adding the acyl azide compound and 2,5-dihydroxy-1,4-dithiane into an organic solvent for ring closure reaction to obtain a reaction solution.
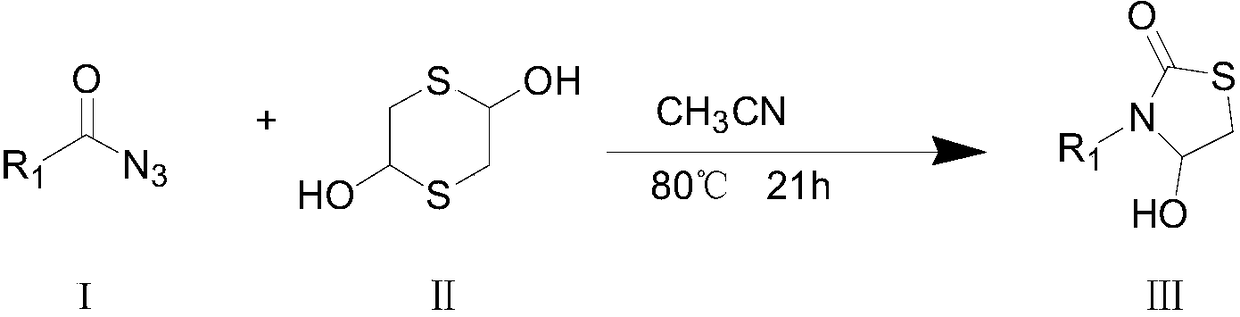
[0031] The synthetic route of the present invention is as follows:
[0032]
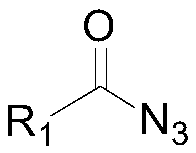
[0033] Wherein, "I" is the structural formula of the acyl azide compound, R 1 It is the No. 3 substituent in the 4-hydroxyl-3-substituted-thiazolidin-2-one compound to be prepared. In this embodiment, R 1 Preferred are phenyl, 4-methylphenyl, 4-chlorophenyl, 4-bromophenyl, 4-trifluoromethylphenyl, 3-nitrophenyl, 4-cyanophenyl, 2-thiophene ...
Embodiment 1
[0048] Add 220.5 mg (1.5 mmol) of benzoyl azide to the reaction flask, then add 4 ml of acetonitrile and stir to dissolve at room temperature, then add 125.4 mg (0.825 mmol) of 2,5-dihydroxy-1,4 dithiane to In the reaction flask, add 4ml of acetonitrile, after the addition is complete, stir at 80°C, during which the reaction progress is detected by TLC (developing agent is petroleum ether-ethyl acetate (5:1, v / v)), when TLC detects the reaction When the result is that the benzoyl azide disappears, it means that the reaction is over.
[0049] After the reaction, the solid was removed by filtration, and extracted with ethyl acetate-water. After the obtained organic layer was washed and dried, the residual solvent was distilled off by a rotary evaporator to obtain a concentrate.
[0050] Select 10g of 200-300 mesh silica gel as the column chromatography, use petroleum ether: ethyl acetate=5:1 (v / v) as the eluent, set the flow rate as 3ml / min to the concentrated liquid column chro...
Embodiment 2
[0056] Add 220.5 mg (1.5 mmol) of benzoyl azide to the reaction flask, then add 3 ml of toluene and stir to dissolve at room temperature, then add 125.4 mg (0.825 mmol) of 2,5-dihydroxy-1,4 dithiane to In the reaction flask, add 3ml of toluene, after the addition is complete, stir at 80°C, during which the reaction progress is detected by TLC (the developer is petroleum ether-ethyl acetate (1:1, v / v)), when TLC detects the reaction When the result is that the benzoyl azide disappears, it means that the reaction is over.
[0057] After the reaction, the solid was removed by filtration, and extracted with ethyl acetate-water. After the obtained organic layer was washed and dried, the residual solvent was distilled off by a rotary evaporator to obtain a concentrate.
[0058] Select 10g of 200-300 mesh silica gel as the column chromatography, use petroleum ether:ethyl acetate=10:1 (v / v) as the eluent, set the flow rate to 3ml / min for the concentrated liquid column chromatography, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com