Mutant of L-amino acid oxidase
An amino acid and oxidase technology, applied in the field of genetic engineering, can solve the problem of low yield and achieve the effect of single target product, easy control and improved catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of L-amino acid oxidase mutant
[0029] Using the genome of Corynebacterium glutamicum as a template, primers were designed for PCR, and the gene encoding L-amino acid oxidase derived from Corynebacterium glutamicum was cloned. The primers used were: as the forward primer, with Obtain the target gene for the amplification of the reverse primer (the bold parts are the restriction sites of XhoⅠ and hindⅢ respectively); PCR program: pre-denaturation at 95°C for 3 minutes, denaturation at 95°C for 3 minutes, and then enter the following cycle: denaturation at 98°C for 10 seconds, 55°C Anneal for 30s, extend at 72°C for 1min and 40s; 34 cycles; extend at 72°C for 10min, hold at 4°C.
[0030] Digest the target gene and expression vector pET28a with restriction endonuclease XhoI and hindIII at 37°C for 2 hours, then use T4 ligase to digest and gel-recover the target gene and plasmid pET28a at 16°C for 10 hours
[0031] Using the pET28a plasmid (abbr...
Embodiment 2
[0041] Example 2 Induced expression of L-amino acid oxidase mutant Q183A
[0042] The mutant Q183A-pET28a was transformed into Escherichia coli BL21(DE3) cells, and the transformants were selected for sequencing verification. The verified positive transformants were cultured overnight at 37°C in LB medium, then transferred to TB medium with 2% inoculum, and cultured to OD 600 When =0.6-0.8, add IPTG with a final concentration of 0.04mM, and induce at 25°C for 12h.
[0043] Collect the fermentation broth, centrifuge at 8000rpm for 8min at 4°C to collect the cells.
Embodiment 3
[0044] Embodiment 3: the catalytic efficiency of recombinant bacteria to L-valine
[0045] The catalytic reaction system is 20mL, including recombinant E. coli whole cells with a final concentration of 30g / L, substrate 70g / L-valine, and 20mM Tris buffer (pH=8.0), and reacted at 200rpm and 25°C for 24h After sampling, the samples were analyzed by HPLC. Catalytic efficiency is calculated from the amount of product formed.
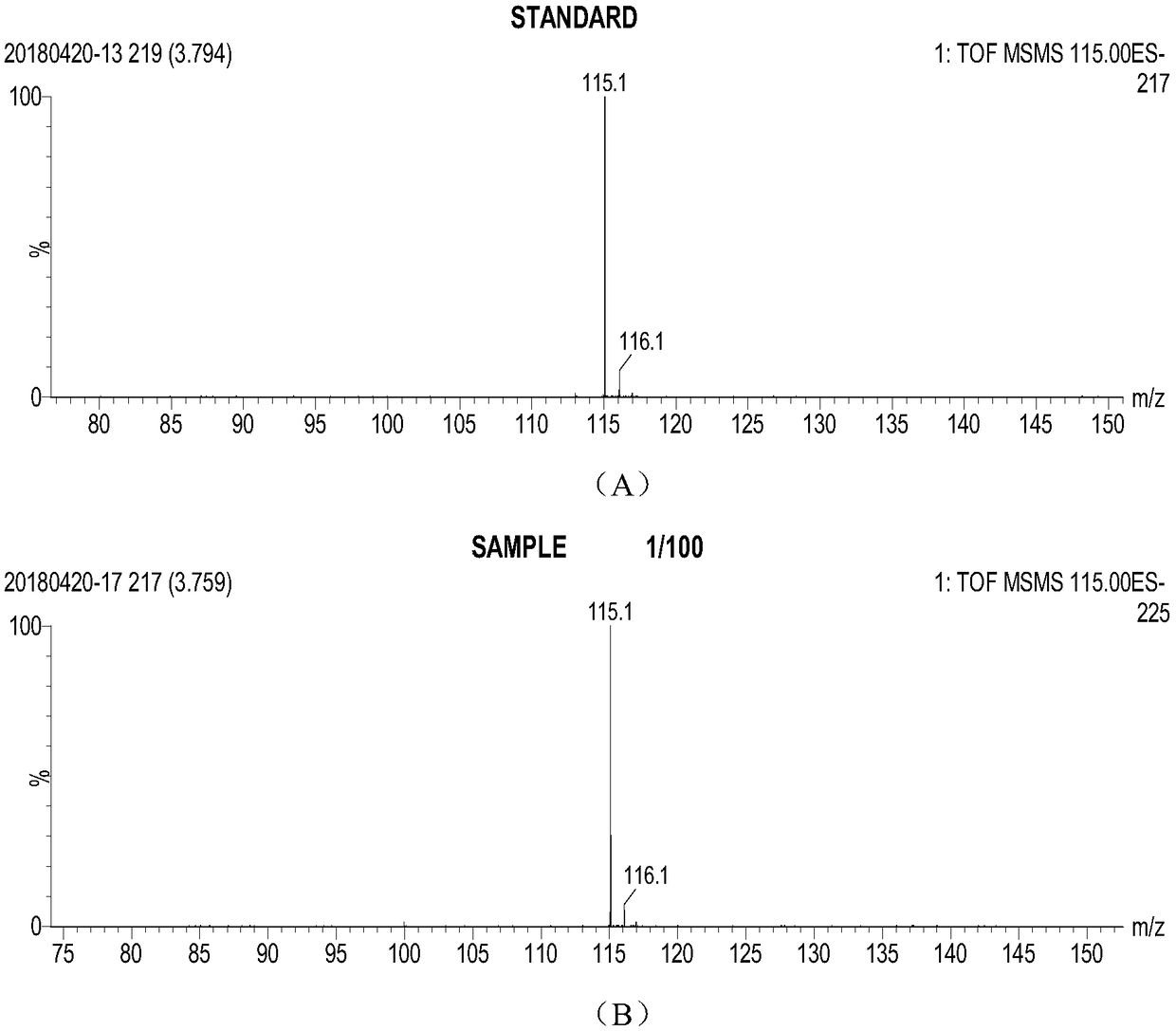
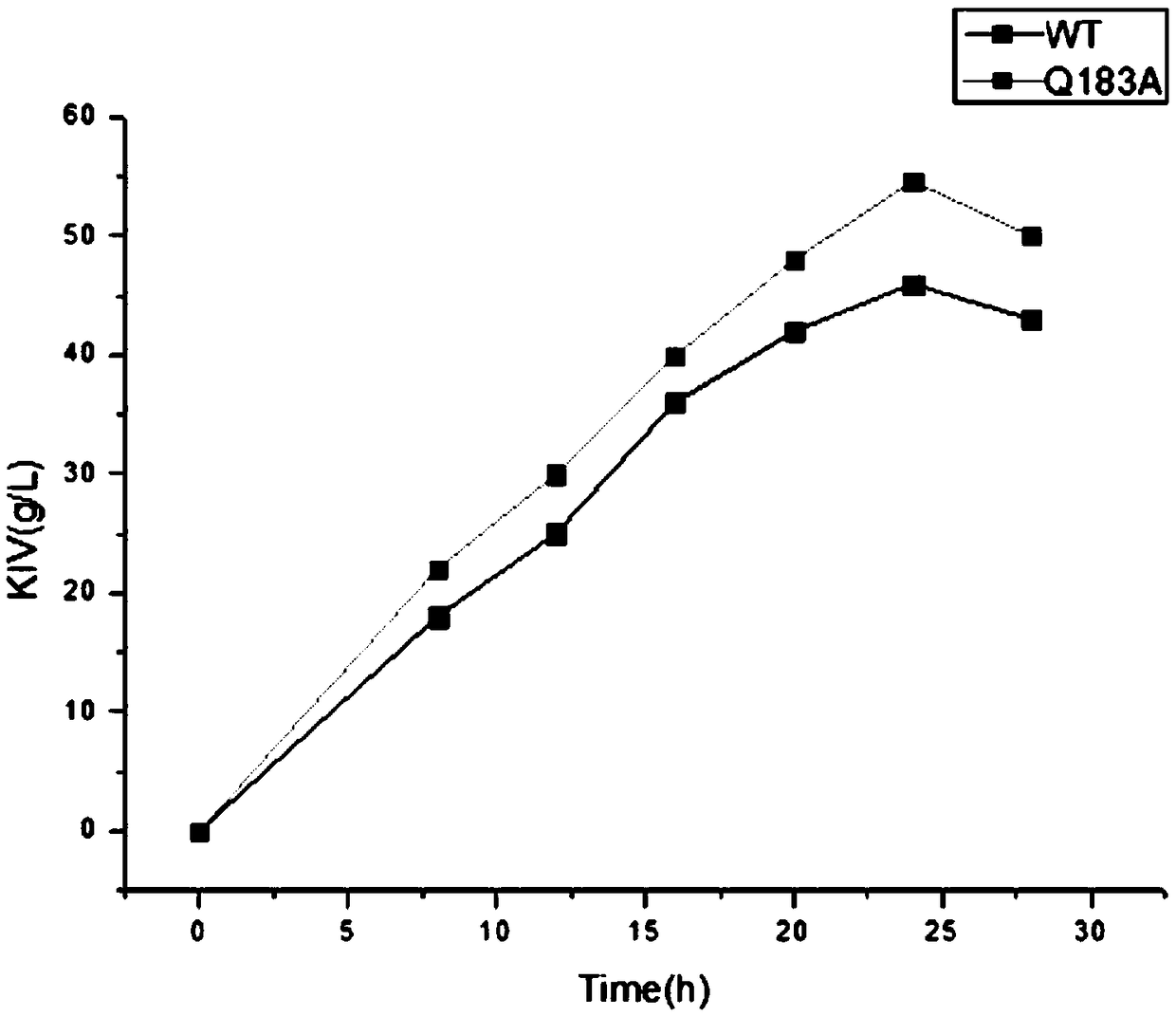
[0046] The recombinant bacteria expressing the L-amino acid oxidase mutant Q183A catalyzed L-valine to obtain the mass spectrum of the reaction product as shown in figure 1 As shown, according to the comparison of the mass spectrograms of the ketovaline standard substance and the reaction solution, it is proved that the reaction product is ketovaline. Catalytic results such as figure 2 As shown, the yield of recombinant bacteria expressing L-amino acid oxidase mutant Q183A can reach 54.6g / L after catalysis for 24 hours, and the conversion rate reaches 84....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com