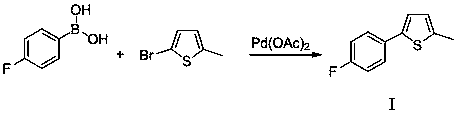Synthesis method of canagliflozin
A synthesis method and methyl technology, applied in the field of medicine, can solve problems such as serious environmental pollution, and achieve the effects of low price, easy availability and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
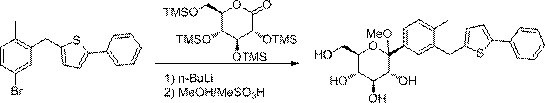
[0037] The synthetic method of canagliflozin of the present invention, take 4-fluorophenylboronic acid as starting material and 2-methyl-5-bromothiophene to couple and synthesize 2-methyl-5-(4-fluorophenyl) thiophene, then Bromo, and 4-bromotoluene by Friedel-Crafts alkylation reaction to synthesize 2-(2-methyl-5-bromobenzyl)-5-(4-fluorophenyl)thiophene, and then with 2,3,4, 6-Tetra-O-trimethylsilyl-D-gluconolactone is condensed, detrimethylsilyl-protected, etherified, and demethoxylized to obtain the hypoglycemic drug canagliflozin; specifically, it includes the following steps:
[0038] (1) Preparation (1) of 2-methyl-5-(4-fluorophenyl) thiophene: 4-fluorophenylboronic acid and 2-methyl-5-bromothiophene are mixed according to molar ratio 1:1.1-1.3, with Water is a solvent, tetrabutylammonium bromide is a phase transfer catalyst, catalyzed by palladium acetate, and 2-methyl-5-(4-fluorophenyl)thiophene (I) is synthesized by reaction at room temperature; the specific reaction i...
Embodiment 1
[0049] Example 1 Preparation of 2-methyl-5-(4-fluorophenyl)thiophene (I)
[0050] Take 140g of 4-fluorophenylboronic acid, 176g of 2-methyl-5-bromothiophene, 325g of tetrabutylammonium bromide, 2.24g of palladium acetate, 300g of potassium carbonate, 2000g of deionized water, and stir at room temperature for 2h. , extracted with 400 ml of ethyl acetate, dried, filtered, recovered ethyl acetate under reduced pressure, and recrystallized from 70% ethanol to obtain 178.5 g of white crystals, with a yield of 93%.
Embodiment 2
[0051] Example 2 Preparation of 2-bromomethyl-5-(4-fluorophenyl)thiophene (II)
[0052] Take 96g of 2-methyl-5-(4-fluorophenyl)thiophene, 100g of N-bromosuccinimide, 200ml of chloroform, 2.4g of BPO, and react at 70°C for 6h. After the reaction, the system is cooled to room temperature , filtered, the filtrate was washed with saturated brine, washed with water, dried, filtered, and the solvent was recovered under reduced pressure, and the residue was recrystallized with petroleum ether: ethyl acetate (1:1) to obtain 129.6 g of a light yellow solid, with a yield of 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com