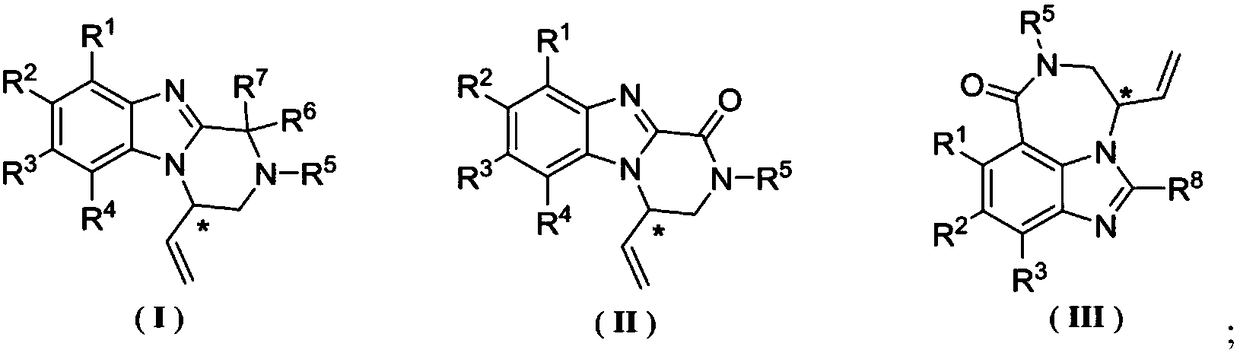
Benzimidazole chiral heterocyclic compound as well as preparation method and application thereof
A technology of benzimidazoles and heterocycles is applied in the field of chiral compound synthesis to achieve the effect of high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117]Example 1.O,O'-[(R)-(1,1'-binaphthyl-2,2'-diphenolyl)]-N-[(R)-2-methyl-1,2, Preparation of 3,4-tetrahydroquinolinyl] phosphoramidite (L5):
[0118] Under nitrogen protection, in a dry 250mL three-neck flask, add 50mL of toluene and phosphorus trichloride (0.67mL, 7.7mmol), and cool to 0°C; in another dry 50mL eggplant-shaped bottle, add (R) -2-Methyl-1,2,3,4-tetrahydroquinoline (1.13g, 7.7mmol), toluene (8mL), and triethylamine (1.8mL, 12.9 mmol), then gradually added dropwise to the above 250mL in the three-necked flask. After the dropwise addition, the temperature was raised to 80°C for 6 hours, and then gradually cooled to -78°C. A solution of (R)-binaphthalene (7.7 mmol) and triethylamine (3.5 mL, 25.2 mmol) in toluene (30 mL) and tetrahydrofuran (6 mL) was slowly added to the system. The system was stirred overnight at room temperature, filtered through celite, and the solvent was removed under reduced pressure. The crude product was separated and purified by col...
Embodiment 2
[0122] Example 2. (S)-[6,6'-((R,R)-2,4-pentanedioloxy)]-2,2'-N,N-bis[(1R)-1-benzene Preparation of ethyl ethyl] phosphoramidite (L12):
[0123] Under nitrogen protection, phosphorus trichloride (32.30 μ L, 0.37 mmol), triethylamine (257.90 μ L, 1.85 mmol), bis[(1R)-1-phenylethyl]amine ( 83.70μL, 0.37mmol), anhydrous toluene (20mL), and then stirred at 50°C for 4h. Cool to room temperature, add (S)-[6,6'-((2R,4R)-2,4-pentanedioloxy)]-2,2'-dihydroxybiphenyl (105.86mg, 0.37mmol) Tetrahydrofuran solution (20 mL) was stirred at room temperature for 16 h. The crude product was purified by column chromatography to obtain the target compound.
[0124] (S)-[6,6-(2R,4R-Pentadioxy)]-(1,1')-biphenyl-(3,5-dioxa-4-phosphacyclohepta[e,g] [1,4])-bis ((R)-1-phenylethyl)amine (L12)
[0125]
[0126] White solid, yield: 84%; melting point: 109.6–110.3°C; [α] D 27 =+59 (c=0.1, CHCl 3 ). 1 H NMR (400MHz, CDCl 3 )δ7.31–7.25(m,2H),7.16–7.10(m,2H),6.98(dd,J=13.6,8.2Hz,2H),6.89(t,J=7.5Hz,...
Embodiment 3
[0127] Example 3. (R)-[6,6'-((S,S)-2,3-butanedioloxy)]-2,2'-N-[(R)-2-methyl-1 , Preparation of 2,3,4-tetrahydroquinolinyl] phosphoramidite (L13):
[0128] In a dry 250mL three-necked flask, add (R)-[6,6'-((S,S)-2,3-butanedioloxy)]-2,2'-dihydroxybiphenyl ( 2.00g, 7.3mmol) and toluene (50mL), phosphorus trichloride (9.60mL, 110.00mmol) and N-methylpyrrolidone (21.00μL, 0.22mmol) were added under nitrogen protection respectively, reacted at 50°C for 30min, and then The reaction liquid was cooled to room temperature, and then the phosphorus trichloride was distilled off under reduced pressure, and then the concentrated phosphorous oxychloride solid was dissolved with 50 mL of tetrahydrofuran; in another dry 250 mL round bottom flask, add (R)-2- Methyl-1,2,3,4-tetrahydroquinoline hydrochloride (1.20g, 6.60mmol) and 30 mL of tetrahydrofuran were cooled to -78°C, and then n-butyl lithium ( 2.5mol / L, 5.30mL), after the dropwise addition, continue to stir and react for 1h; finally, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com