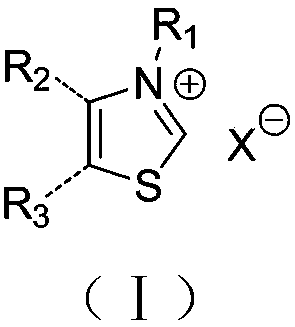
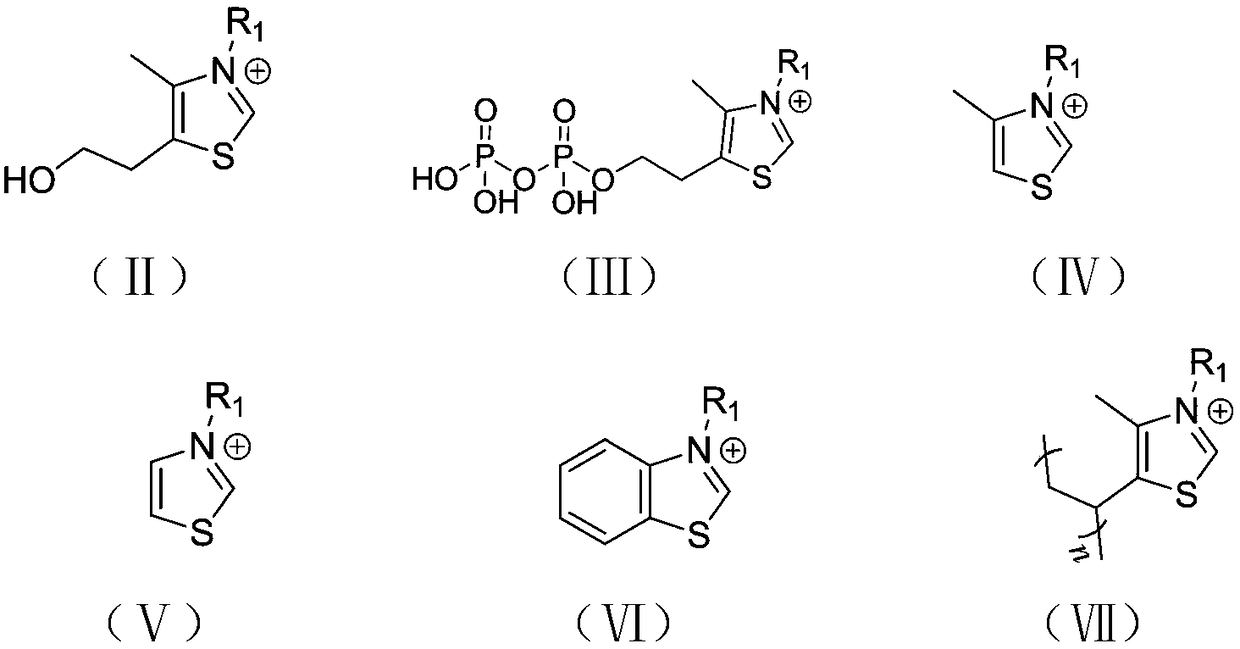
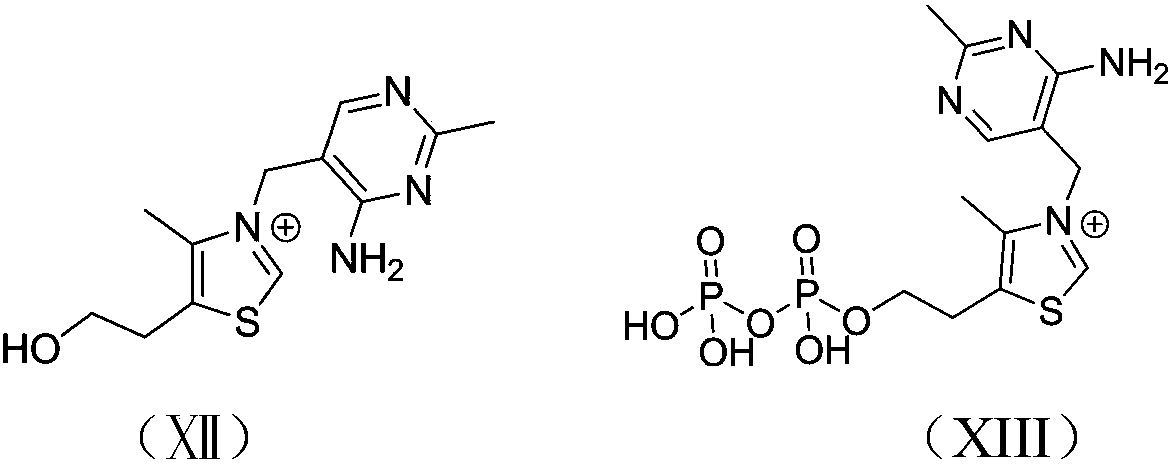
Cyclic ester bulk ring-opening polymerization method by taking thiazole onium salt as catalyst and catalyst preparation method
A thiazolium salt and ring-opening polymerization technology, which is applied in the direction of carboxylate preparation, sulfonate preparation, chemical instruments and methods, etc., can solve the problem of harsh conditions for the preparation of nitrogen-heterocyclic carbene, low catalytic efficiency of thiazole-based carbene, and inability to Catalytic ring-opening polymerization and other problems, to achieve good catalytic ability, easy and stable storage and use, and good catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Dissolve 3.37g of thiamine hydrochloride in 5ml of water to make solution A, dissolve 0.40g of sodium hydroxide in 2ml of water to make solution B, dissolve 2.87g of lithium bistrifluoromethanesulfonylimide in 2ml of water, and make It is solution C; mix A and B solutions, and after fully stirring, the hydrochloric acid in thiamine hydrochloride is basically neutralized; then add solution C to the mixed solution of A and B, fully stir and let it stand until the precipitation is complete; filter, Dry to obtain white powdery solid i.
[0036] Using i as a catalyst, the molar ratio of caprolactone to catalyst is 100 / 1 (caprolactone 2.85g, catalyst 0.137g) After feeding, close the reaction system, stir fully in an oil bath at 150°C for 12h, then stop heating; dissolve the product in 20ml of dichloromethane, after filtering, add the filtrate dropwise to 80ml of ethanol, stir fully after the dropwise addition, let it stand until the precipitation is complete, filter out the p...
Embodiment 2
[0038] Dissolve 3.37g of thiamine hydrochloride in 5ml and water to make solution A, dissolve 0.40g of sodium hydroxide in 2ml and water to make solution B, dissolve 1.68g of sodium hexafluorophosphate in 2ml and water to make solution C; mix A and B solutions, after fully stirring, the hydrochloric acid in thiamine hydrochloride is basically neutralized; then add solution C to the mixed solution of A and B, stir fully and let stand until the precipitation is complete; filter and dry to obtain white Powdered solid ii.
[0039] Use ii as the catalyst, the molar ratio of caprolactone to catalyst is 100 / 1 (caprolactone 2.85g, catalyst 0.103g) After feeding, close the reaction system, stir fully in an oil bath at 150°C for 12h, then stop heating; dissolve the product in 20ml of dichloromethane, after filtering, add the filtrate dropwise to 80ml of ethanol, stir fully after the dropwise addition, let it stand until the precipitation is complete, filter out the precipitation, and dr...
Embodiment 3
[0041] Dissolve 3.37g of thiamine hydrochloride in 5ml and water to make solution A, dissolve 0.40g of sodium hydroxide in 2ml and water to make solution B, dissolve 1.10g of sodium tetrafluoroborate in 2ml and water to make solution C; mix A and B solutions, after fully stirring, the hydrochloric acid in thiamine hydrochloride is basically neutralized; then add solution C to the mixed solution of A and B, stir fully and let stand until the precipitation is complete; filter and dry to obtain white Powdered solid iii.
[0042] Use iii as the catalyst, the molar ratio of caprolactone to catalyst is 100 / 1 (caprolactone 2.85g, catalyst 0.088g) After feeding, close the reaction system, stir fully in an oil bath at 150°C for 12h, then stop heating; dissolve the product in 20ml of dichloromethane, after filtering, add the filtrate dropwise to 80ml of ethanol, stir fully after the dropwise addition, let it stand until the precipitation is complete, filter out the precipitation, and dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com