Pullulanase mutant and application thereof
A pullulanase, mutant technology, applied in the application, enzyme, hydrolase and other directions, to achieve the effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Recombinant bacteria construction
[0032] According to Anoxybacillus sp.WB42 (Wang J, Liu Z, Zhou Z. Cloning and characterization of a novel thermophilic amylopullulanase with a type Ipullulanase structure from Anoxybacillus sp.WB42[J]. 2018, 70 (5-6): 1700265.doi: 10.1002 / star.201700265) the base sequence of the pullulanase is shown in SEQ ID NO.2, and the cloning primers for the designed pullulanase are shown in Table 1 , The plasmid used to construct Escherichia coli is pET-22b(+), with Nde I, XhoI restriction sites. The pullulanase was cloned between the Nde I and Xho I restriction sites of the vector pET-22b(+) to obtain the recombinant plasmid pET-22b(+)-pulA. Proceed as follows:
[0033] (1) Design of pullulanase cloning primers;
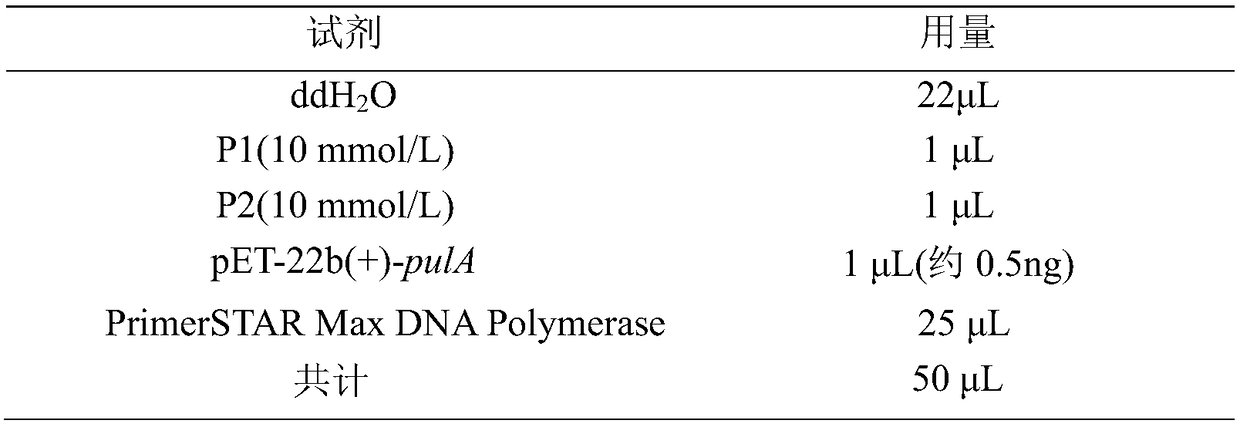
[0034] (2) The gene pulA was cloned by PCR using Anoxybacillus sp.WB42 as a template;
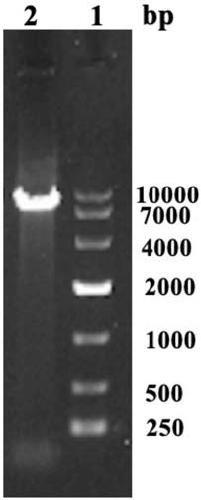
[0035] (3) Recovering and purifying the above-mentioned PCR product, performing double digestion on the purified PCR product an...
Embodiment 2
[0037] Embodiment 2: the preparation of pullulanase mutant
[0038] (1) Single mutant
[0039] According to the gene sequence of pullulanase as shown in SEQ ID NO.2, primers for introducing mutant K419R were designed and synthesized, and fast PCR technology was used to carry the recombinant plasmid pET-22b encoding the gene of wild-type pullulanase (+)-pulA was used as a template, and the pullulanase gene was subjected to site-directed mutation, the DNA coding sequence was determined, and the 419th lysine codon was identified as an arginine codon, and the single mutant pullulanase K419R was obtained .
[0040] The site-directed mutagenesis primers for introducing the K419R mutation are:
[0041] The nucleotide sequence is the forward primer of SEQ ID NO.3:
[0042] 5'-GCATTCTCGATATTGACACGATG CGT GAAGTC-3' (the underline is the mutated base)
[0043] The nucleotide sequence is the reverse primer of SEQ ID NO.4:
[0044] 5'-ACAACTGCCTCGACTTC ACG CATCGTGTC-3' (the underl...
Embodiment 3
[0062] Embodiment 3: fermentation and purification of pullulanase
[0063] Pick the recombinant strain E.coli BL21(DE3) / pET-22b(+)-pulA expressing wild-type pullulanase and inoculate it in 5ml LB medium (containing 100μg / mL ampicillin antibiotic), 37℃, 200r / Incubate overnight with shaking. The overnight culture was inoculated into 100 mL LB medium (containing 100 μg / mL ampicillin antibiotic) at an inoculum size of 1% (v / v), and cultured at 37°C with shaking at 200 r / min until OD 600 At 0.6-0.8, add 10 μM inducer IPTG, induce at 16°C for 16-18 hours to obtain bacterial cells, and collect recombinant bacterial cells by centrifugation at 4°C and 8000r / min.
[0064] The above-mentioned recombinant cells collected by centrifugation were dissolved in 20 mL of binding buffer solution, ultrasonically disrupted, and after centrifugation at 13,000 g for 25 min, the supernatant was filtered with a 0.22 μm filter membrane. Then equilibrate the 1mL His TrapHP column with 10 times the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com