Method for detecting 5-Isoquinolinesulfonic acid in fasudil hydrochloride
A technology of ethyl isoquinolinesulfonate and fasudil hydrochloride, which is applied in the field of detection of ethyl 5-isoquinolinesulfonate in fasudil hydrochloride, can solve the problem of 5-isoquinolinesulfonate Ethyl acetate detection method, ineffective detection, long running time and other problems, to achieve the effect of high detection sensitivity, good for safety control, and short running time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]1. Instruments and materials
[0043] 1. Main instruments
[0044] Mass spectrometer: AB SCIEX company, model API 4000.
[0045] Liquid chromatograph: Shimadzu company, model LC20AD.
[0046] Electronic balance: METTLER company, XP205 type.
[0047] 2. Experimental materials
[0048] Ethyl 5-isoquinolinesulfonate reference substance: provided by Kunming Pharmaceutical Group Co., Ltd., with a purity of 97.62%.
[0049] Fasudil hydrochloride API: Kunming Pharmaceutical Group Co., Ltd., batch number 201201-01.
[0050] 2. Experimental method
[0051] 1. Solution preparation
[0052] 1.1 0.1% formic acid-water solution: precisely pipette 1ml of formic acid into 1000ml of water, and mix well.
[0053] 1.2 Diluent: Measure 300ml of water and 700ml of acetonitrile, mix well.
[0054] 1.3 Reference substance stock solution (50ng / ml): Take about 2mg of 5-isoquinolinesulfonate reference substance, accurately weigh it, put it in a 200ml measuring bottle, dissolve it with di...
Embodiment 2
[0100] Embodiment 2, adopt the inventive method to carry out quality analysis to Fasudil hydrochloride
[0101] Fasudil hydrochloride raw material is from Kunming Pharmaceutical Group Co., Ltd., with 3 batches in total, and the batch numbers are 201201-01, 201201-02, and 201201-03.
[0102] The preparation of need testing solution (0.5mg / ml): get fasudil hydrochloride crude drug 10mg, accurately weighed, put in 20ml measuring bottle, dissolve and dilute to scale with diluent, shake up, as need testing solution .
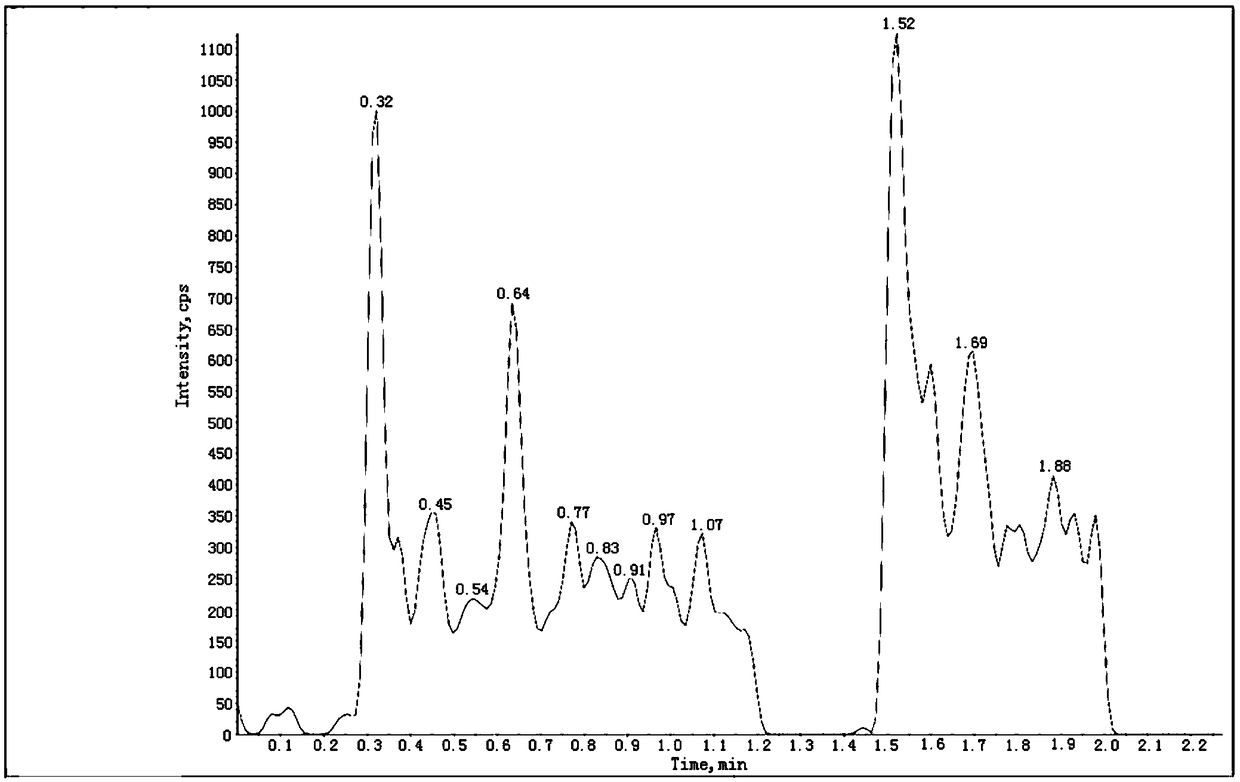
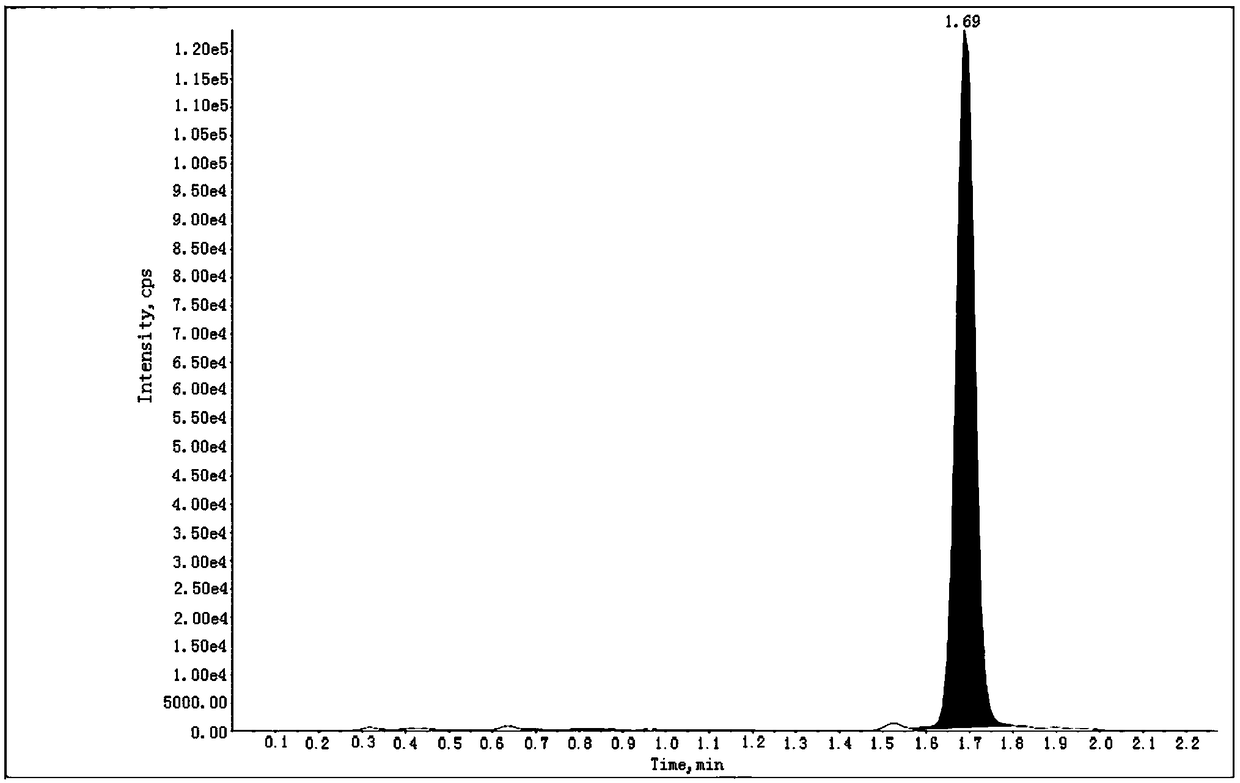
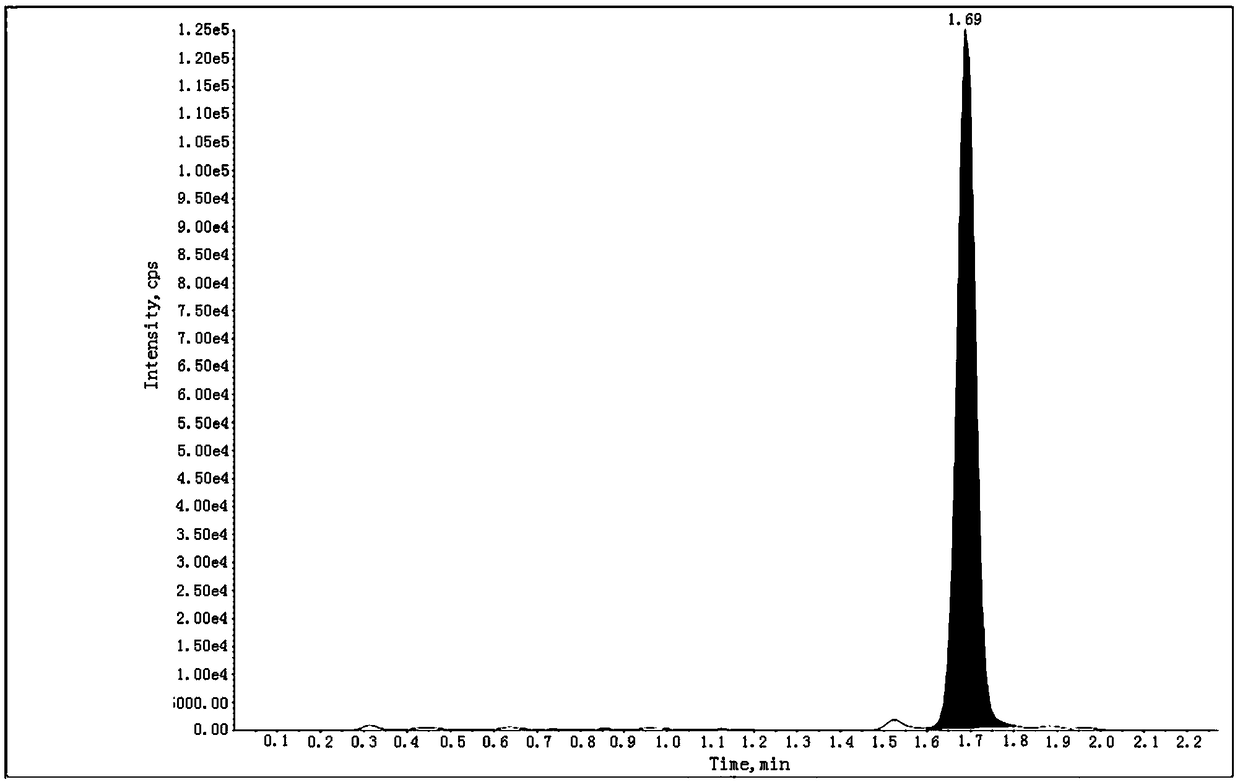
[0103] Get the above-mentioned need testing solution and inject high performance liquid chromatography and use triple quadrupole mass spectrometer in conjunction, record chromatogram (the collection of illustrative plates of 201201-01 batch sees Figure 4 , the spectrum of batch 201201-02 and Figure 4 Similar, for the spectrum of batch 201201-03 see Figure 5 ), calculate the content of genotoxic impurity ethyl 5-isoquinolinesulfonate in the test product accordin...
Embodiment 3
[0108] This embodiment is with embodiment 1, and difference is that the concentration of fasudil hydrochloride need testing solution is 0.4mg / ml, and the concentration of ethyl 5-isoquinolinesulfonate reference substance solution is 0.1ng / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com