Near-infrared window II emission type fluorescent dye as well as preparation method and application thereof
A fluorescent dye, near-infrared technology, used in luminescent materials, organic dyes, azo dyes, etc., can solve the problems of reduced molar extinction coefficient, fluorescence quenching, limited performance, etc., to achieve a large molar extinction coefficient, not easy to lyochromic , The effect of wide adjustable range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
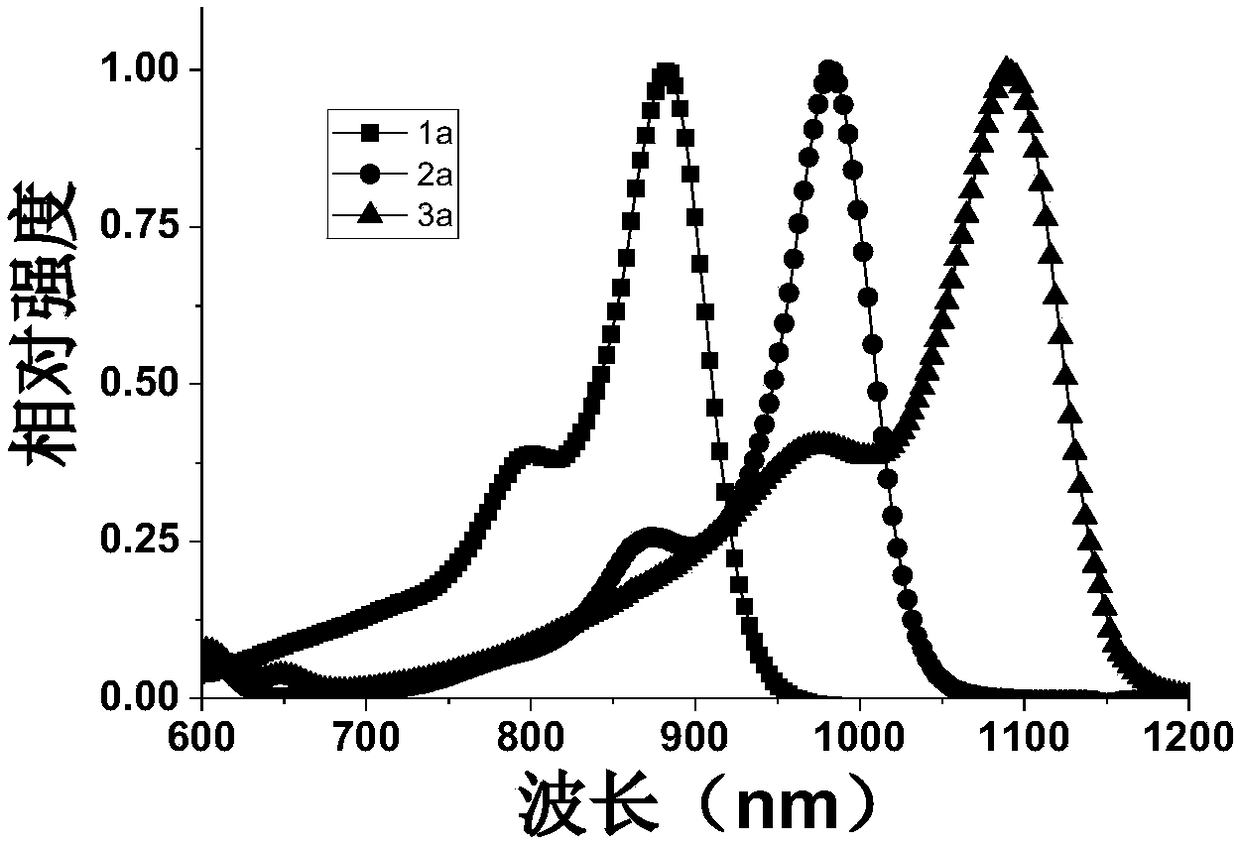
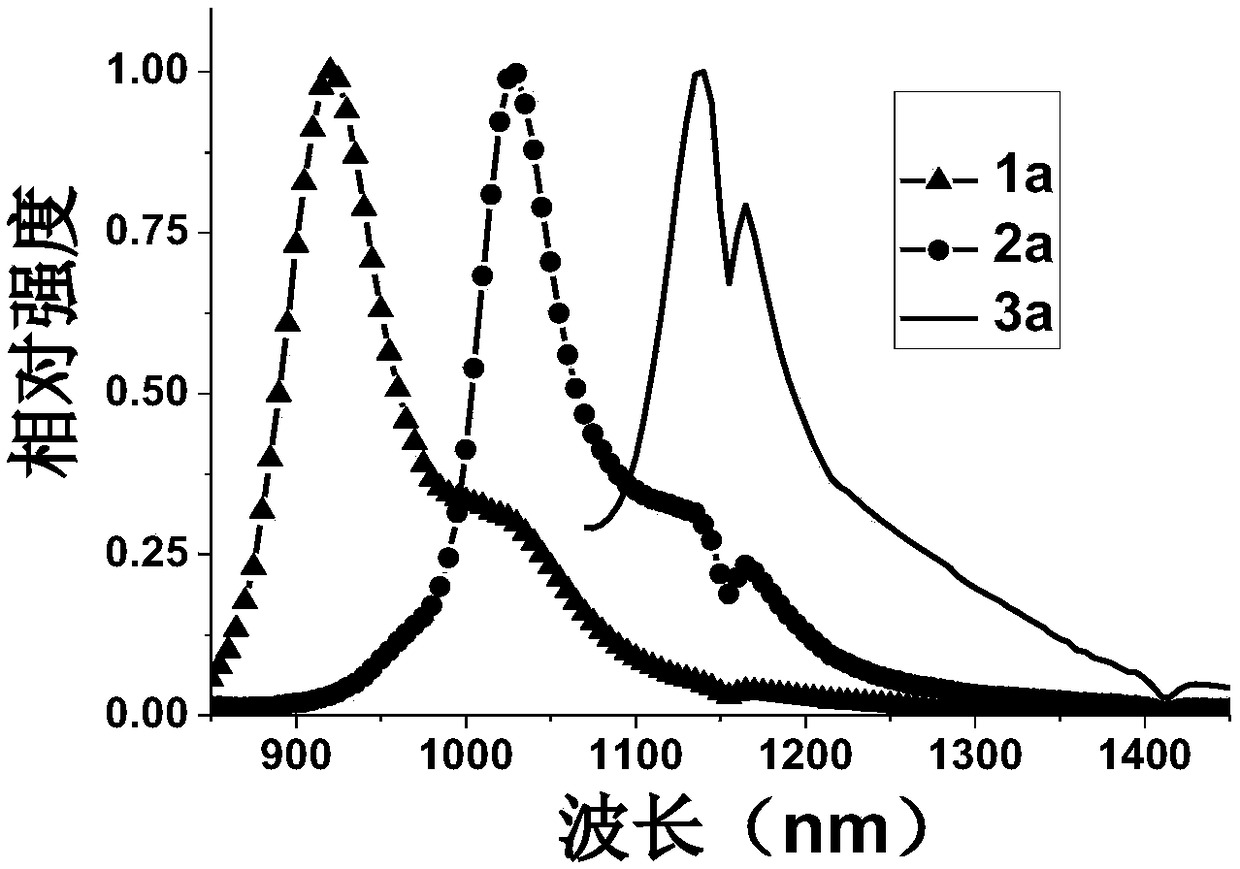
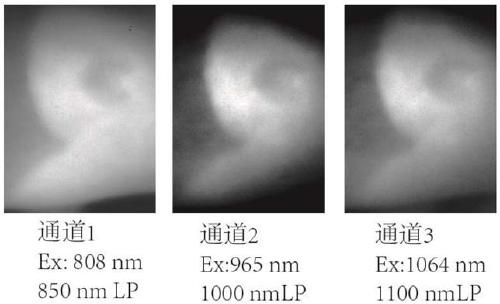
[0037] The preparation of near-infrared fluorescent dye 1a, the compound structural formula is as follows:
[0038]
[0039] Concrete synthetic route is as follows:
[0040]
[0041] Concrete synthetic steps are as follows:
[0042] Compound 4 (237 mg, 0.5 mmol), compound 5 (177.5 mg, 0.5 mmol), paraformaldehyde (15 mg, 0.5 mmol), sodium acetate (41 mg, 0.5 mmol) were mixed in 10 mL of acetic anhydride, under nitrogen Under protection, react at 80°C for 1 hour. After the reaction was completed, filter while hot, dissolve the filter cake with dichloromethane, and separate by column chromatography (dichloromethane / methanol=100 / 1, v / v) to obtain the final fluorescent dye 1a with a yield of 50%. 1 H NMRδ8.02 (d, J = 6.28 Hz, 1H), 7.57 (t, J = 7.24 Hz, 1H), 7.51 (t, J = 7.56 Hz, 1H), 7.47 (s, 1H), 7.16 (d, J = 7.48Hz, 1H), 6.87 (d, J = 8.16 Hz, 1H), 6.60 (d, J = 8.92 Hz, 1H), 6.46 (s, 1H), 6.41 (d, J = 8.92 Hz, 1H), 6.30 (d, J = 7.76 Hz, 2H), 6.25 (s, 1H), 3...
Embodiment 2
[0044] The preparation of near-infrared fluorescent dye 2a, the compound structural formula is as follows:
[0045]
[0046] Concrete synthetic route is as follows:
[0047]
[0048] The specific synthesis steps are as follows
[0049] Intermediate 5 (177.5 mg, 0.5 mmol), malondialdehyde dianiline hydrochloride (64.7 mg, 0.25 mmol), sodium acetate (41 mg, 0.5 mmol) were mixed in 10 mL of acetic anhydride, under nitrogen protection, The reaction was carried out at 80° C. for 2 hours. After the reaction was completed, the solvent was spin-dried by a rotary evaporator, and then 50 mL of water was added and extracted with dichloromethane. The organic phase was concentrated and separated by column chromatography (dichloromethane / methanol=100 / 1, v / v) to obtain the final fluorescent dye 2a , yield 67%. 1 H NMRδ 7.81 (d, J =12.20 Hz, 2H), 7.39 (s, 2H), 7.35 (d, J = 8.72 Hz, 2H), 6.81 (d, J = 8.12 Hz,2H), 6.78 (s, 2H), 6.43 (t, J = 12.20 Hz, 1H), 3.48-3.45 (m, 8H), 2.6...
Embodiment 3
[0051] The preparation of near-infrared fluorescent dye 3a, the compound structural formula is as follows:
[0052]
[0053] Concrete synthetic route is as follows:
[0054]
[0055] The specific synthesis steps are as follows
[0056] Intermediate 5 (177.5 mg, 0.5 mmol), pentadiene acetal diphenylamine hydrochloride (71 mg, 0.25 mmol), sodium acetate (41 mg, 0.5 mmol) were mixed in 10 mL of acetic anhydride, under nitrogen protection , reacted at 80°C for 2 hours. After the reaction was completed, the solvent was spin-dried by a rotary evaporator, and then 50 mL of water was added and extracted with dichloromethane. The organic phase was concentrated and separated by column chromatography (dichloromethane / methanol=100 / 1, v / v) to obtain the final fluorescent dye 3a , yield 61%. 1 H NMR δ 7.68 (d, J = 9.36 Hz, 2H), 7.43 (s, 2H), 7.39 (d, J = 8.96 Hz, 2H), 7.31 (t, J = 12.72Hz, 1H), 6.84 (dd, J = 8.84 Hz, 1.88 Hz, 2H), 6.69 (d, J = 1.88 Hz, 2H), 6.64(d, J = ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com