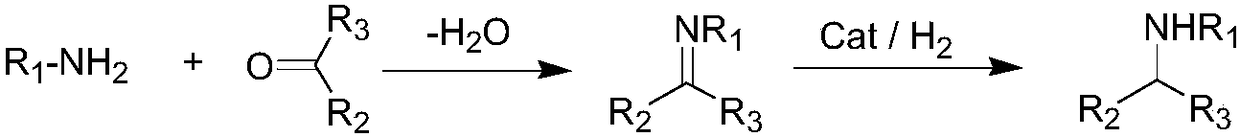
Method for preparing chiral amine by asymmetric hydrogenation of imine
A hydrogenated imine, asymmetric technology, applied in asymmetric synthesis, organic chemical methods, cyanide reaction preparation, etc., can solve the problems of difficult ligand synthesis, harsh reaction conditions, low reactivity, etc., and achieve good industrial application value. , Easy to operate, simple to synthesize effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
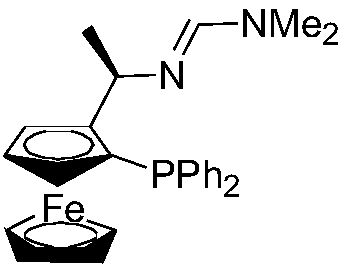
[0035] 1 Preparation of ligand ferrocenephosphine-dimethylamidine imine
[0036] Dissolve (R)-1-[(α-amino)ethyl]-(S)-2-(diphenylphosphino)ferrocene (10mmol) in 15ml N,N-dimethylformamide dimethyl In the acetal, the reaction was stirred at room temperature until the raw material point basically disappeared (TLC detection), and the volatile components were removed under reduced pressure, and then recrystallized with n-hexane to obtain orange square flaky crystals. The structural formula of ferrocenephosphine-dimethylamidine imine is as follows:
[0037]
[0038] The NMR spectrum of ferrocenephosphine-dimethylamidine imine:
[0039] 1 H NMR (400MHz, CDCl 3 ):δ7.52-7.07(m,11H),4.60(s,1H),4.56(m,1H),4.25(s,1H),3.97(s,5H),3.70(s,1H),2.21( s,6H),1.56(d,J=6.8Hz,3H); 31 P NMR (162MHz, CDCl 3 ): δ-22.8.
[0040] 2 Catalyst Preparation
[0041] The metal precursor [Ir(COD)Cl] of 6.717g 2 And 9.367g of the ligand was added in 20L of dichloroethane, stirred at room temperature ...
Embodiment 2
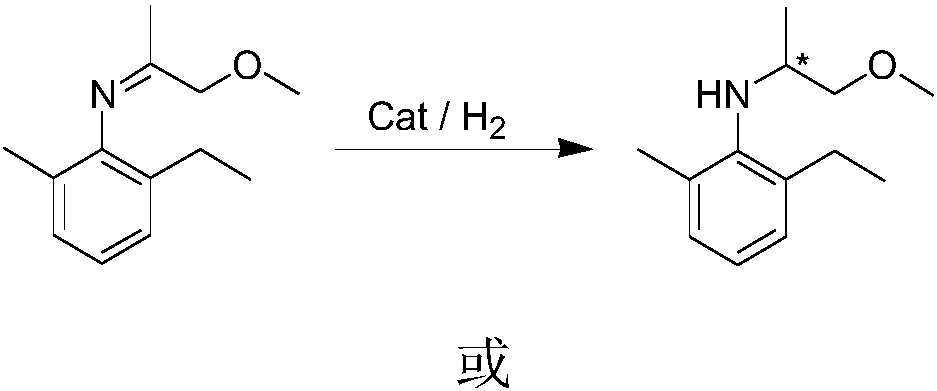
[0048] The reaction pressure was 10 bar, other conditions were the same as in Example 1, the reaction conversion rate was greater than 95% by GC analysis, and 80% ee by HPLC analysis.
Embodiment 3
[0050] The reaction temperature was 25° C., and other conditions were the same as in Example 1. The reaction conversion rate was 90% by GC analysis and 81% ee by HPLC analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com