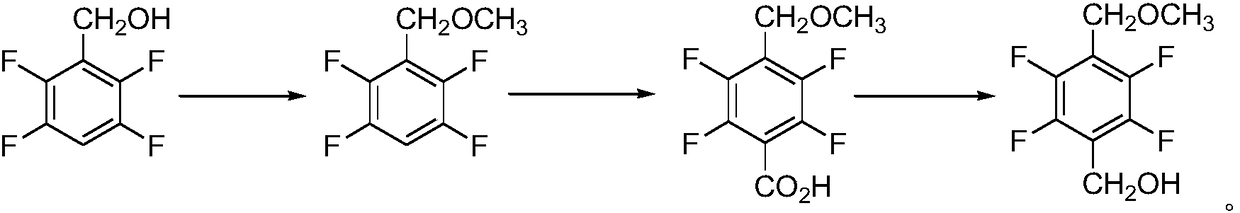
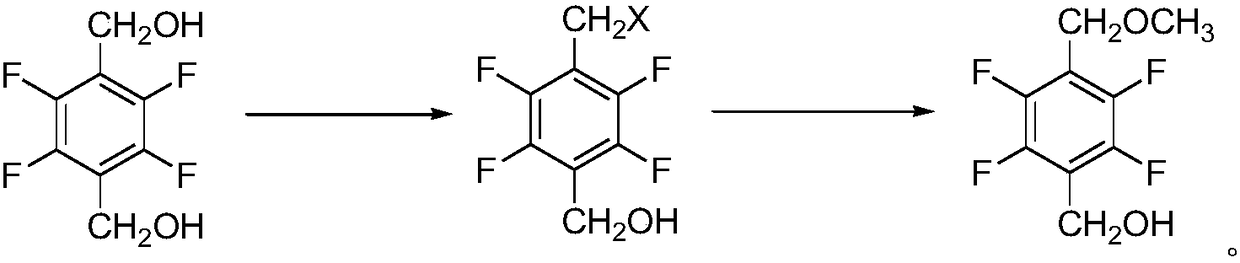
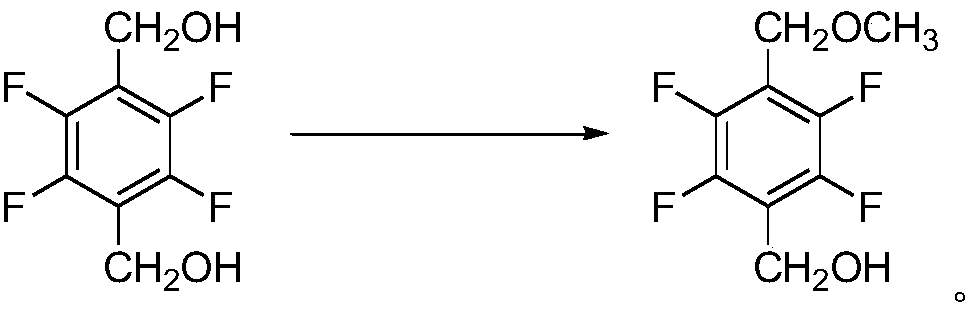
Synthesis method of 2,3,5,6-tetrafluoro-4-methoxymethyl benzyl alcohol
一种甲氧基甲基苯甲醇、甲氧基甲基的技术,应用在有机合成领域,能够解决未发现应用价值、反应收率下降、合成成本上升等问题,达到社会经济价值显著、产品质量好、操作简单的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Add 140 grams of compound (I), 420 grams of 70% sulfuric acid aqueous solution, and 1120 grams of propionic acid into a 2-liter reaction flask, stir and heat up to 140-145 ° C for 10 hours, stop the reaction, and cool down. The reaction solution was extracted with 700 grams of chloroform, the organic phases were combined, and the chloroform was removed by distillation under reduced pressure to obtain 159.5 grams of mixture (II) (HPLC detection: 2,3,5,6-tetrafluoro-4-methoxymethylbenzyl alcohol Propionate 35.5%, 2,3,5,6-Tetrafluorotere-xylylenedimethanol Dipropionate 20.2%, 2,3,5,6-Tetrafluorotere-xylylenedimethanol Monopropionate 16.8%, 2, 3,5,6-Tetrafluorotere-xylylenedimethanol 4.8%, 2,3,5,6-Tetrafluoro-4-methoxymethylbenzyl alcohol 11.8%, and 1,4-bis(methoxymethyl) -2,3,5,6-tetrafluorobenzene 9.1%), for the next reaction.
[0049] The resulting mixture (II) was added to a 2-liter reaction flask, 1250 grams of water and 370 grams of potassium carbonate were added, st...
Embodiment 2
[0052] Add 150 grams of compound (I), 600 grams of 60% sulfuric acid aqueous solution, and 900 grams of acetic acid into a 2-liter reaction flask, stir and heat up to 120-125° C. for 8 hours, stop the reaction, and cool down. The reaction solution was extracted with 960 grams of toluene, and the organic phases were combined to obtain 1125 grams of a toluene solution of the mixture (II) (HPLC detection: 2,3,5,6-tetrafluoro-4-methoxymethylbenzyl alcohol acetate 32.5 %, 2,3,5,6-Tetrafluorotere-xylylenedimethanol diacetate 22.4%, 2,3,5,6-Tetrafluorotere-xylylenedimethanol monoacetate 17.8%, 2,3,5, 6-tetrafluoro-tere-phenylenedimethanol 5.4%, 2,3,5,6-tetrafluoro-4-methoxymethylbenzyl alcohol 11.6% and 1,4-bis(methoxymethyl)-2,3 , 5,6-Tetrafluorobenzene 8.2%), for the next reaction.
[0053]Add the toluene solution of the obtained mixture (II) into a 2-liter reaction flask, add 480 grams of water and 84 grams of potassium hydroxide, stir and heat up to 60-65° C. for 8 hours, stop t...
Embodiment 3
[0056] Add 160 grams of compound (I), 1280 grams of 80% sulfuric acid aqueous solution, and 480 grams of acetic acid into a 2-liter reaction flask, stir and heat up to 110-115° C. for 9 hours, stop the reaction, and cool down. The reaction solution was extracted with 350 grams of dichloroethane, and the organic phases were combined to obtain 525 grams of a dichloroethane solution of the mixture (II) (HPLC detection: 2,3,5,6-tetrafluoro-4-methoxymethyl Benzyl Alcohol Acetate 33.4%, 2,3,5,6-Tetrafluoro-tere-xylylene Dimethanol Diacetate 22.3%, 2,3,5,6-Tetrafluoro-Tetrafluoro-Tere-Phenyl Dimethanol Monoacetate 18.1%, 2,3,5,6-Tetrafluoro-tere-phenylenedimethanol 3.4%, 2,3,5,6-tetrafluoro-4-methoxymethylbenzyl alcohol 14.3% and 1,4-bis(methoxymethyl base)-2,3,5,6-tetrafluorobenzene 6.8%) for the next reaction.
[0057] Add the dichloroethane solution of the obtained mixture (II) into a 2-liter reaction flask, add 1000 grams of water and 240 grams of sodium carbonate, stir and rais...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com