Complex of bifunctional connecting agent realizing core coordination with carbonyl metal and preparation method thereof
A technology of bifunctional linker and metal carbonyl, which can be applied to the 7/17 group organic compounds without C-metal bonds, preparations for in vivo tests, isotope-introduced organic compounds, etc. fit and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
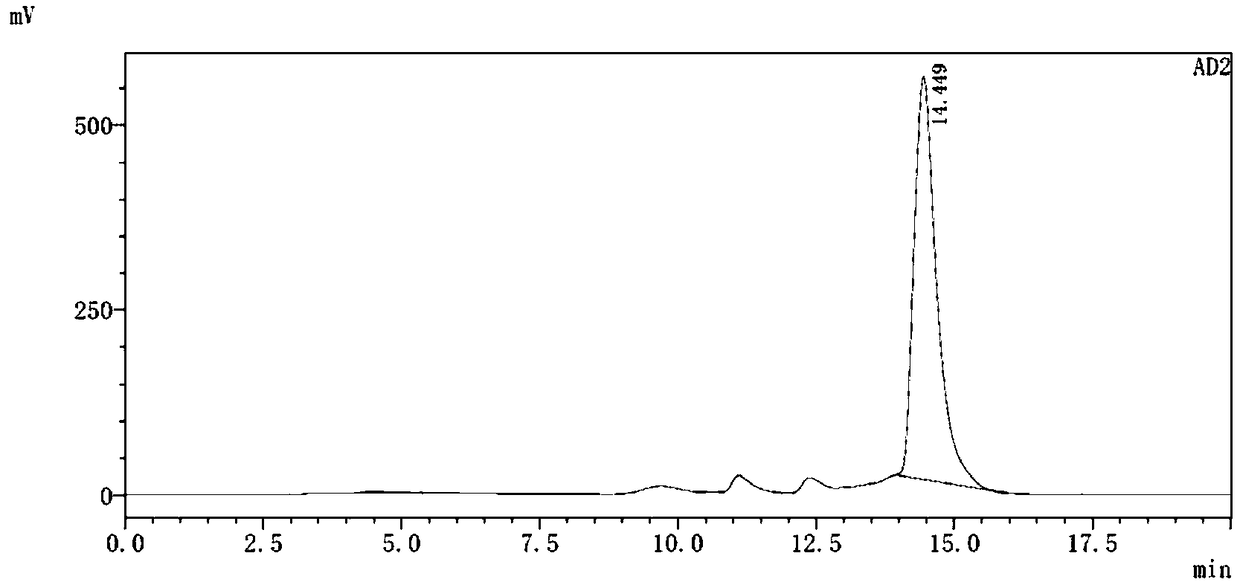
[0074] 99m Tc(CO) 3 -L 2 Preparation of complexes:
[0075] (1) Ligand L 2 Synthesis
[0076] Synthesis of compound 3 (methyl 3-(4-hydroxyphenyl)propionate): p-hydroxyphenylpropionic acid (3g, 18.1mmol) and 50mL methanol were added to the round-bottomed flask, followed by the dropwise addition of trifluoro Boronium diethyl ether (0.3mL) was stirred at room temperature for 6 hours, and the organic phase in the filtrate was removed under reduced pressure using a rotary evaporator, and separated by a silica gel column with n-hexane / ethyl acetate (v / v, 2 / 8). Collect target component, remove solvent under reduced pressure, obtain 2.72g white solid (yield: 84%);
[0077] 1 HNMR (400MHz, CDCl 3 )δ: 7.07(d, 2H, J=8.4Hz), 6.76(d, 2H, J=8.4Hz), 4.72(s, 1H), 3.68(s, 3H), 2.89(t, 2H, J=7.6 Hz), 2.60 (t, 2H, J=7.6Hz); HRMS (ESI) theoretical molecular weight C 10 h 13 o 3 (M+H) + , 181.0865; measured molecular weight, 181.0815;
[0078] Synthesis of compound 4 (methyl 3-(3-form...
Embodiment 2
[0098] 99m Tc(CO) 3 -L 3 Preparation of complexes:
[0099](1) Ligand L 3 preparation of
[0100] 1) Double activated (HBED-CC) TFP 2 Synthesis:
[0101] From compound 1 (N, N'-bis[2-hydroxyl-5-(carboxyethyl) benzyl]ethylenediamine-N, N'-diacetic acid) as the reaction starting material, compound 1 and ferric chloride Mix and stir at room temperature for 20 minutes according to the equivalent of 1:1.1, and the product is purified by flash chromatography to obtain [Fe(HBEDCC)] – ; to 1000 μL concentration of 0.01M [Fe(HBED-CC)] – Add 10 equivalents of TFP (trifluopyrazine) and 4 equivalents of N, N'-diisopropylcarbodiimide to the DMF solution of DMF, react at room temperature for 3 days, and purify the product by preparative HPLC to obtain [ Fe(HBED-CC)]TFP 2 , after diluting with water, the diluted [Fe(HBED-CC)]TFP 2 The solution was injected onto the RP18 column, and the RP18 filter column was washed with 3mL, 1M hydrochloric acid and washed with 4mL of H 2 O washin...
Embodiment 3
[0115] Re(CO) 3 -L 2 Preparation of complexes
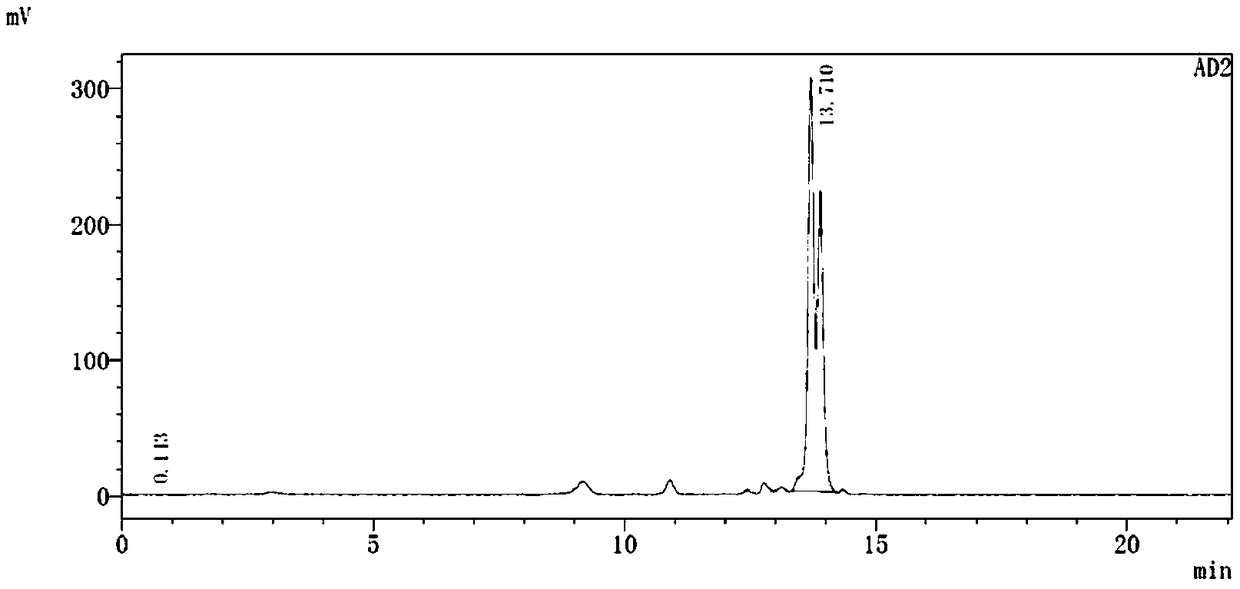
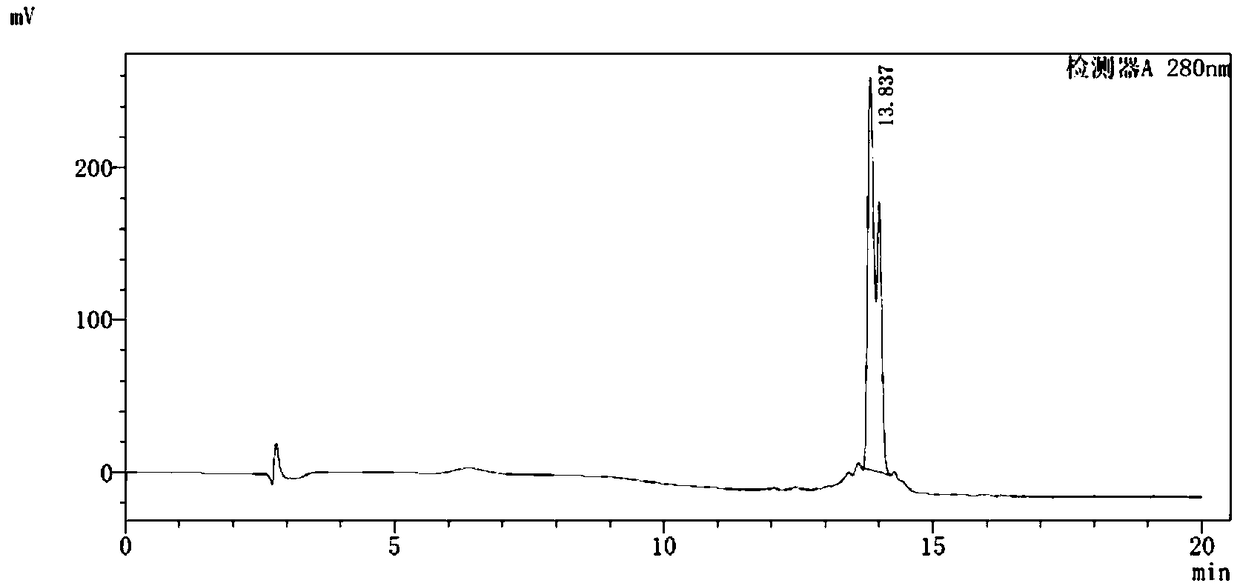
[0116] An aqueous solution of 10ml rhenium pentacarbonyl bromide (80mg, 0.2mmol) was refluxed at 105°C for 24 hours, and then the L 2 (30mg, 0.038mmol) added to containing Re (CO) 3 (H 2 O) 3 In the above reaction solution (2 mL) of the Br intermediate, the mixture was acidified with 0.1 N HCl to pH = 5 and heated at 95 °C for 3 h to give Re(CO) 3 -L 2 complex; its possible structure is as follows, such as image 3 Shown, is the Re(CO) prepared by Example 3 of the present invention 3 -L 2 UV-LC analysis chart. The analysis results show that the product has only one structure, and the existence of split peaks indicates that there are chiral isomers in the structure of the complex.
[0117]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com