Superhydrophobic phase change energy storage material microcapsule and preparation method thereof
A technology of phase-change energy storage materials and microcapsules, which is applied in the field of phase-change energy storage material microcapsules and its preparation, and in the field of phase-change energy storage material microcapsules, which can solve corrosion, unstable chemical properties, and poor thermal conductivity, etc. problems, achieve the effects of improving surface roughness, wide application fields, excellent packaging and protection effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
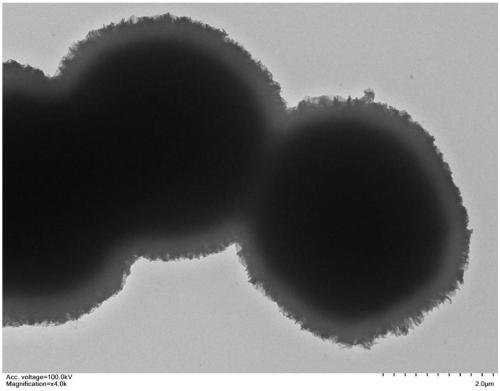
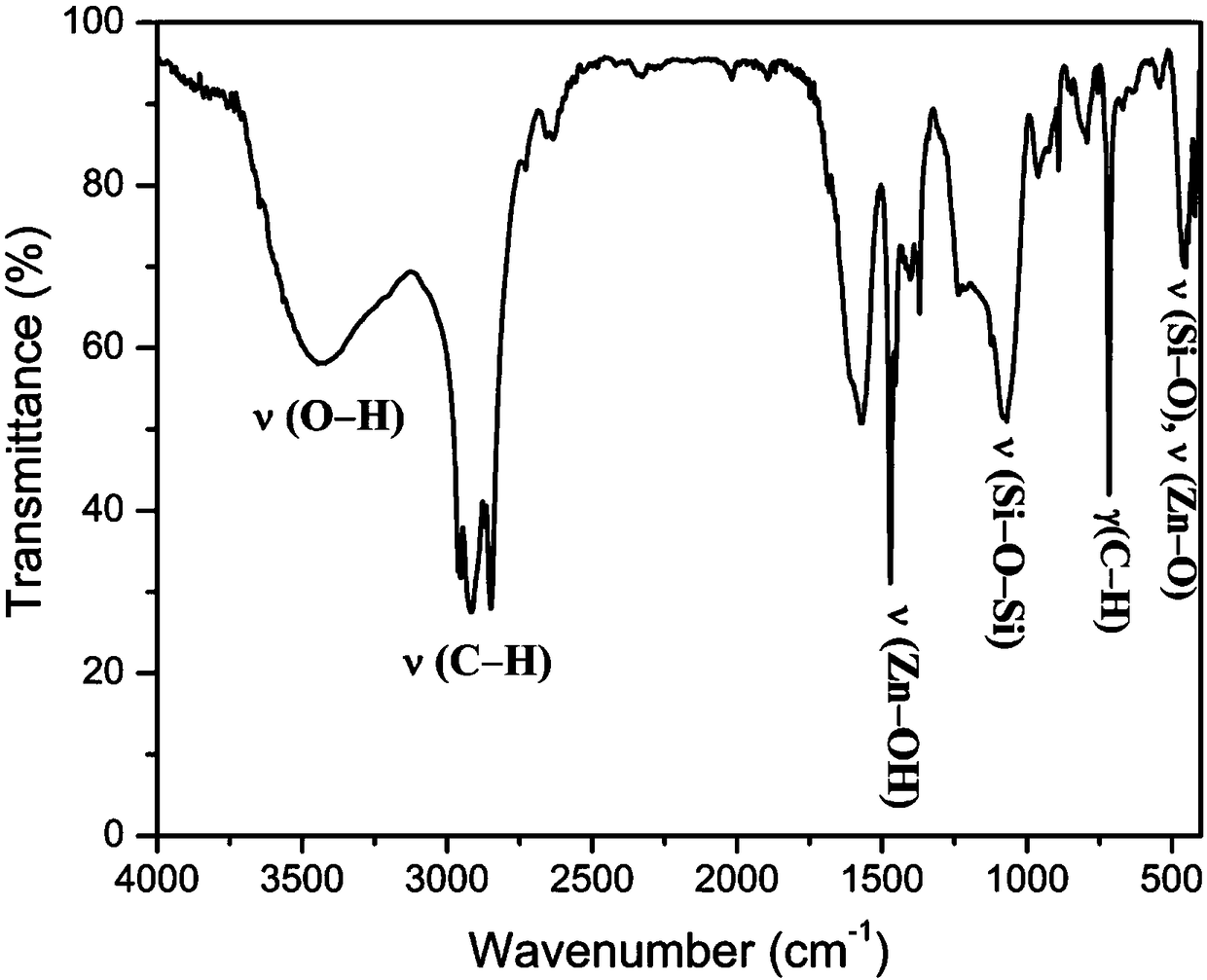
Image
Examples
Embodiment 1
[0035] (1) Synthesis of organic phase change materials coated with inorganic wall materials in the inner layer
[0036] In a 250ml three-necked flask, 5.0g tetraethyl orthosilicate and 5.0g n-docosane were dispersed for 1h at a mechanical stirring rate of 500rpm to form a uniform oil phase; subsequently, 0.8g of cationic surfactant hexadecyl Dissolve trimethylammonium bromide in 60ml of formamide and add it to the three-necked flask, keep the temperature and stirring rate constant to form a uniform and stable oil-in-water emulsion; after emulsifying for 4 hours, slowly add 60ml of 0.8M hydrochloric acid aqueous solution In the mixed emulsion, continue to react at 60°C for 7h, then move to a constant temperature water bath, and age at 50°C for 12h; finally, the product is washed with deionized water, filtered and dried at room temperature to obtain silica phase change material microcapsules.
[0037] (2) Synthesis of zinc oxide shell layer
[0038] 5.0 g of the microcapsules s...
Embodiment 2
[0042] (1) Synthesis of organic phase change materials coated with inorganic wall materials in the inner layer
[0043] In a 250ml three-necked flask, 5.0g of n-butyl titanate and 5.0g of n-docosane were dispersed for 1h at a mechanical stirring rate of 500rpm to form a uniform oil phase; subsequently, 0.5g of anionic surfactant dodecyl Sodium sulfate was dissolved in 60ml of formamide and added to the above-mentioned three-neck flask, keeping the temperature and stirring rate constant to form a uniform and stable oil-in-water emulsion; after emulsification for 4 hours, prepare a mixed solution of 1mL deionized water and 30mL formamide, and Slowly add to the above mixed emulsion, keep the stirring rate constant, continue to stir and react for 8 hours, then move to a constant temperature water bath, and age at 50°C for 12 hours; finally, the product is washed with deionized water, filtered and dried at room temperature to obtain titanium dioxide phase change material micropartic...
Embodiment 3
[0049] (1) Synthesis of organic phase change materials coated with inorganic wall materials in the inner layer
[0050] In a 250ml three-necked flask, 5.0g of zirconium n-propoxide and 5.0g of n-docosane were dispersed at a mechanical stirring rate of 500rpm for 1h to form a uniform oil phase; subsequently, 1.0g of nonionic surfactant Span 60 was dissolved Add 100ml of formamide into the three-necked flask above, keep the temperature and stirring rate constant to form a uniform and stable oil-in-water emulsion; after emulsifying for 4 hours, prepare a mixture of 25mL of deionized water and 25mL of formamide, and slowly add the above In the mixed emulsion, keep the stirring rate constant, continue to stir and react for 8 hours, then move to a constant temperature water bath, and age at 50°C for 12 hours; finally, the product is washed with deionized water, filtered and dried at room temperature to obtain zirconia phase change material microcapsules.
[0051] (2) Synthesis of zi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com