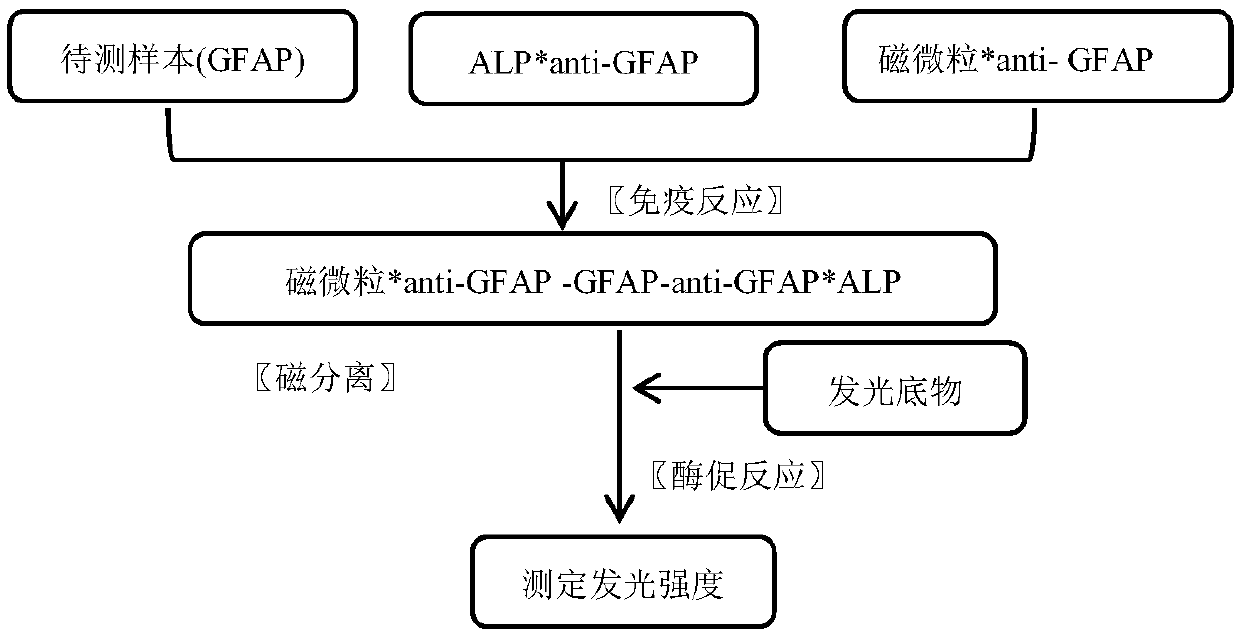
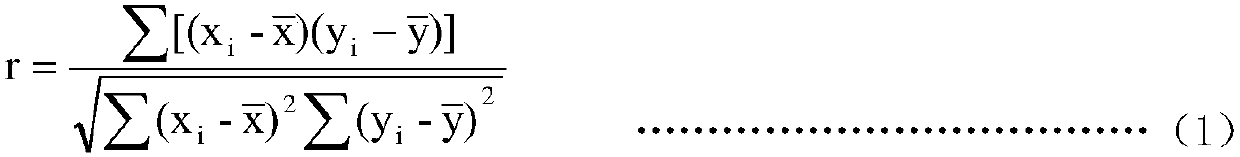
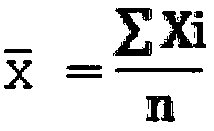Magnetic-particle separation chemiluminescence immunoassay for detecting glial fibrillary acidic protein (GFAP)
A technology of chemiluminescence immunity and glial fiber, which is applied in the direction of chemiluminescence/bioluminescence, and analysis by making materials react chemically, and can solve the problems of low automation of ELISA method, large pollution of RIA method, complicated and cumbersome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Magnetic particle-labeled glial fibrillary acidic protein (GFAP) antibody
[0048] Take 20 mg of carboxyl-modified microparticle solution, settle the magnetic particles with superparamagnetic properties, uniform particle size, and carboxyl (COOH-) active groups on the surface (magnetic separation) under the action of a magnetic field for 10 minutes, remove the supernatant, and settle the magnetic particles Wash 3 times with (2-(N-morpholine)ethanesulfonic acid) MES at a molar concentration of 0.05M in the activation buffer, pH 6.0 buffer solution, 2 ml each time.
[0049] After washing, the magnetic particles were fully suspended with 1.0ml activation buffer (2-(N-morpholine) ethanesulfonic acid) MES with a molar concentration of 0.05M, pH 6.0, and then the activator 1-ethyl-3- [3-dimethylaminopropyl]carbodiimide hydrochloride (EDC), suspension reaction at room temperature for 30 minutes, the molar concentration of EDC is 7.5mM.
[0050] Take 1.0 mg of glial...
Embodiment 2
[0052] Example 2: Alkaline phosphatase (ALP) labeled glial fibrillary acidic protein (GFAP) antibody
[0053] Take 1.0 mg of glial fibrillary acidic protein (GFAP) antibody, concentrate to 2.5 mg / mL, add 5 μL of activator 2-Iminothiolane hydrochloride (2-IT) solution with a concentration of 13.76 mg / mL, and react at room temperature for 15 minutes. Use Sephadex G25 gel column to desalt and collect the activated antibody.
[0054] Take 1.2 mg of alkaline phosphatase (ALP), concentrate it to 2.5 mg / mL, add 12 μL of activator Succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) solution with a concentration of 6.69 mg / mL, and react at room temperature for 15 minute. Use Sephadex G25 gel column to desalt and collect activated alkaline phosphatase (ALP).
[0055] Mix activated glial fibrillary acidic protein (GFAP) antibody and alkaline phosphatase (ALP) according to the ratio of 1.0 mg glial fibrillary acidic protein (GFAP) antibody to 1.0 mg alkaline phosphatase (...
Embodiment 3
[0057] Embodiment 3: Human Tau protein (TAU) magnetic particle separation chemiluminescent immunoassay method
[0058] (1) Immunological reaction: the 50 μl calibrator series of Example 1 (concentrations are respectively 0, 0.8, 2.5, 9.5, 25, 50 ng / ml) were respectively mixed with the quality control substance, 50 μl reagent A of Example 1, Example 1 Add 50 μl of reagent B of 2 to the reaction tube in turn, mix and incubate at 37°C for 15 minutes;
[0059] (2) Magnetic separation: settle the magnetic particles in a magnetic field, remove the supernatant, add 200-500 μl of cleaning solution, remove the magnetic field, then settle the magnetic particles in the magnetic field again, and remove the supernatant; repeat this 2-4 times, To remove unbound antibodies and impurities;
[0060] (3) Reading value: add 150 μl of luminescence substrate solution, after alkaline phosphatase (ALP) catalyzes the luminescence of the substrate, measure the relative luminescence intensity (RLU) wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com