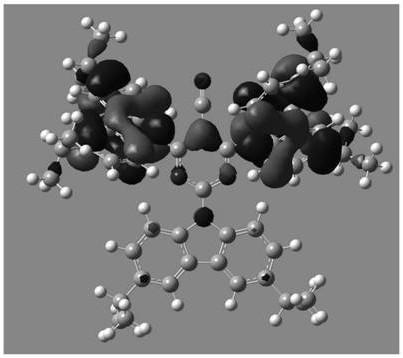
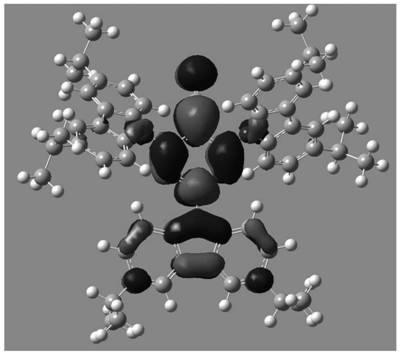
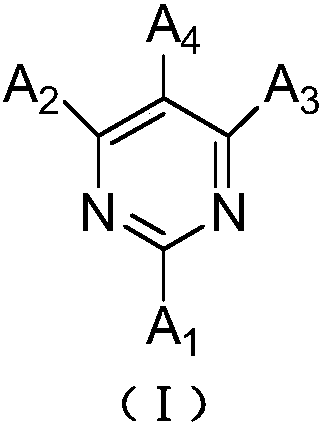
General formula compound and organic light-emitting device
A compound and general formula technology, applied in the field of organic electroluminescent devices, can solve the problems of dependence on global resources and high material costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0075] Synthesis Example 1: Synthesis of Compound P1
[0076]
[0077] Add 8.79g (35mmol) of isopropylcarbazole into a 250ml three-neck flask, add 100ml of N,N-dimethylformamide as a reaction solvent, and stir on a magnetic stirrer for 10min under ice-bath conditions. 0.72g (30mmol) NaH was added to the reaction flask in batches, and the stirring was continued for 1h. Dissolve 2.07g (10mmol) of 2,4,6-trichloro-5-cyanopyrimidine in 40ml of N,N-dimethylformamide solution and add it dropwise to the reaction system. Under reaction 24h. After the reaction, the reaction solution was poured into 200ml concentration of 10% dilute hydrochloric acid to quench, after vacuum filtration, washed with water, dried, the crude product obtained was washed with sherwood oil and dichloromethane (PE:DCM=10: 1) Pass the column as the mobile phase. 5.56 g of white solid powder was obtained with a yield of 65.2%. (1HNMR(δ,CDCl3):7.40(6H,s),7.28(6H,s),6.71-6.68(6H,d),3.08-3.01(6H,m),1.19-1.10(1...
Synthetic example 2
[0078] Synthesis Example 2: Synthesis of Compound P2
[0079]
[0080] Add 8.79g (35mmol) of isopropylcarbazole into a 250ml three-neck flask, add 100ml of N,N-dimethylformamide as a reaction solvent, and stir on a magnetic stirrer for 10min under ice-bath conditions. 0.72g (30mmol) NaH was added to the reaction flask in batches, and the stirring was continued for 1h. Dissolve 2.83g (10mmol) of 2,4,6-trichloro-5-cyanophenylpyrimidine in 40ml of N,N-dimethylformamide solution and add it dropwise to the reaction system. Reaction at room temperature for 24h. After the reaction, the reaction solution was poured into 200ml concentration of 10% dilute hydrochloric acid to quench, after vacuum filtration, washed with water, dried, the crude product obtained was washed with sherwood oil and dichloromethane (PE:DCM=10: 1) Pass the column as the mobile phase. 4.99 g of white solid powder was obtained with a yield of 53.7%.
[0081] (1HNMR(δ,CDCl3):7.60-7.56(4H,d),7.38(6H,s),7.30(6H...
Synthetic example 3
[0082] Synthesis Example 3: Synthesis of Compound P5
[0083]
[0084] Under nitrogen protection, 6.28g (25mmol) isopropylcarbazole, 2.83g (10mmol) 2,4,6-trichloro-5-cyanopyrimidine, 1.92g (20mmol) NaOBu-t, 1.92g (0.2 mmol)(t-Bu) 3 HBF 4 , 0.09g (0.1mmol) Pd 2 (dba) 3 Add it into a 250ml three-neck flask, add 100ml of toluene as a reaction solvent, heat up to reflux temperature, and stir overnight on a magnetic stirrer. After the reaction, the reaction solution was spin-dried, and the obtained crude product was passed through the column with petroleum ether and dichloromethane (PE:DCM=5:1) as the mobile phase. Intermediate 1 was obtained as white solid powder, weighing 4.70 g, and the yield was 73.7%.
[0085] 1.56g (7.5mmol) 9,9-Dimethylacridine Add to a 250ml three-neck flask, add 50ml of N,N-dimethylformamide as a reaction solvent, and stir on a magnetic stirrer for 10min under ice-bath conditions. 0.36g (15mmol) NaH was added to the reaction flask in batches, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com