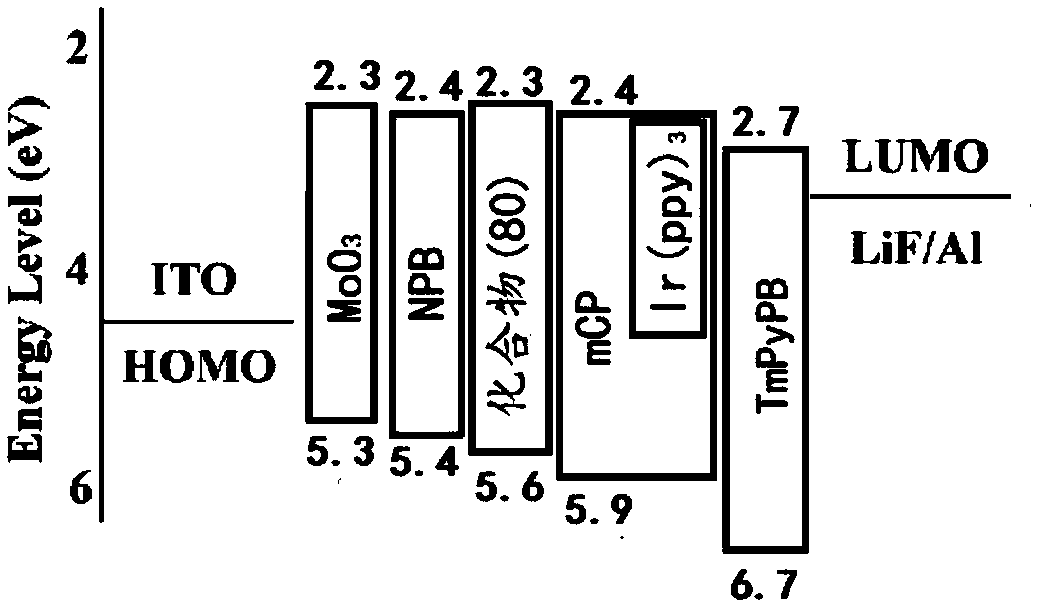
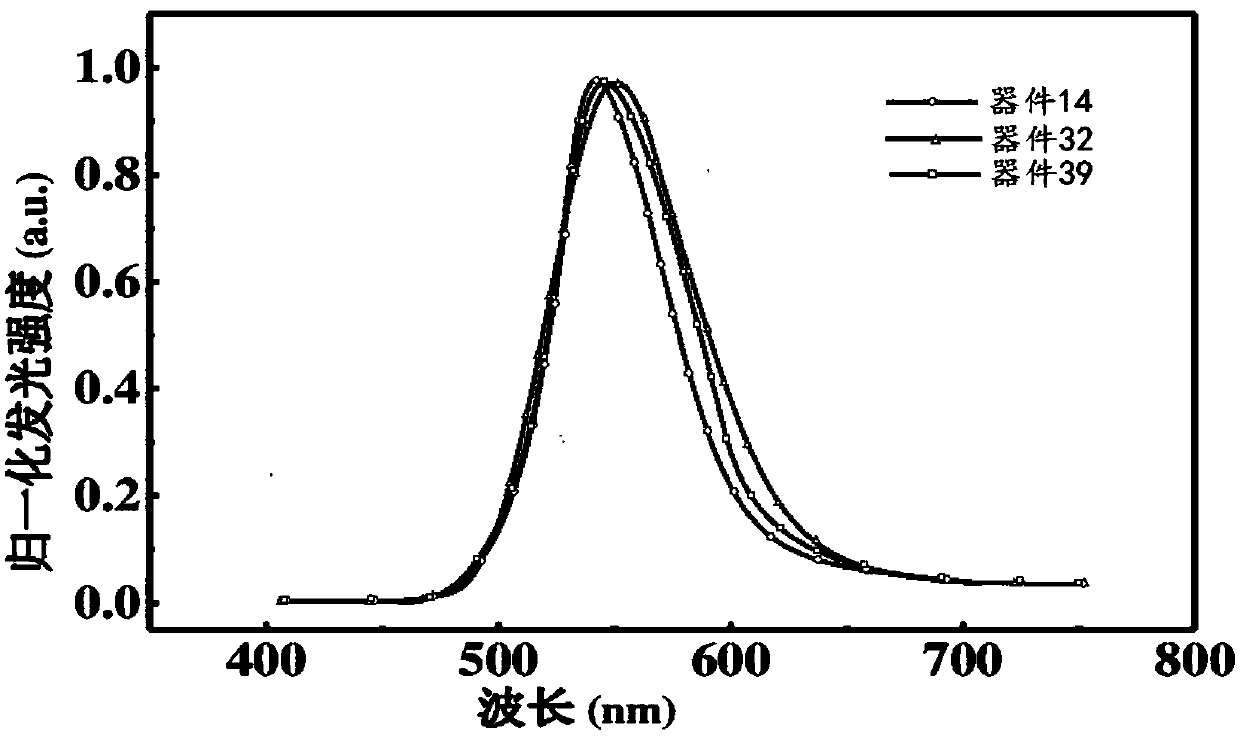
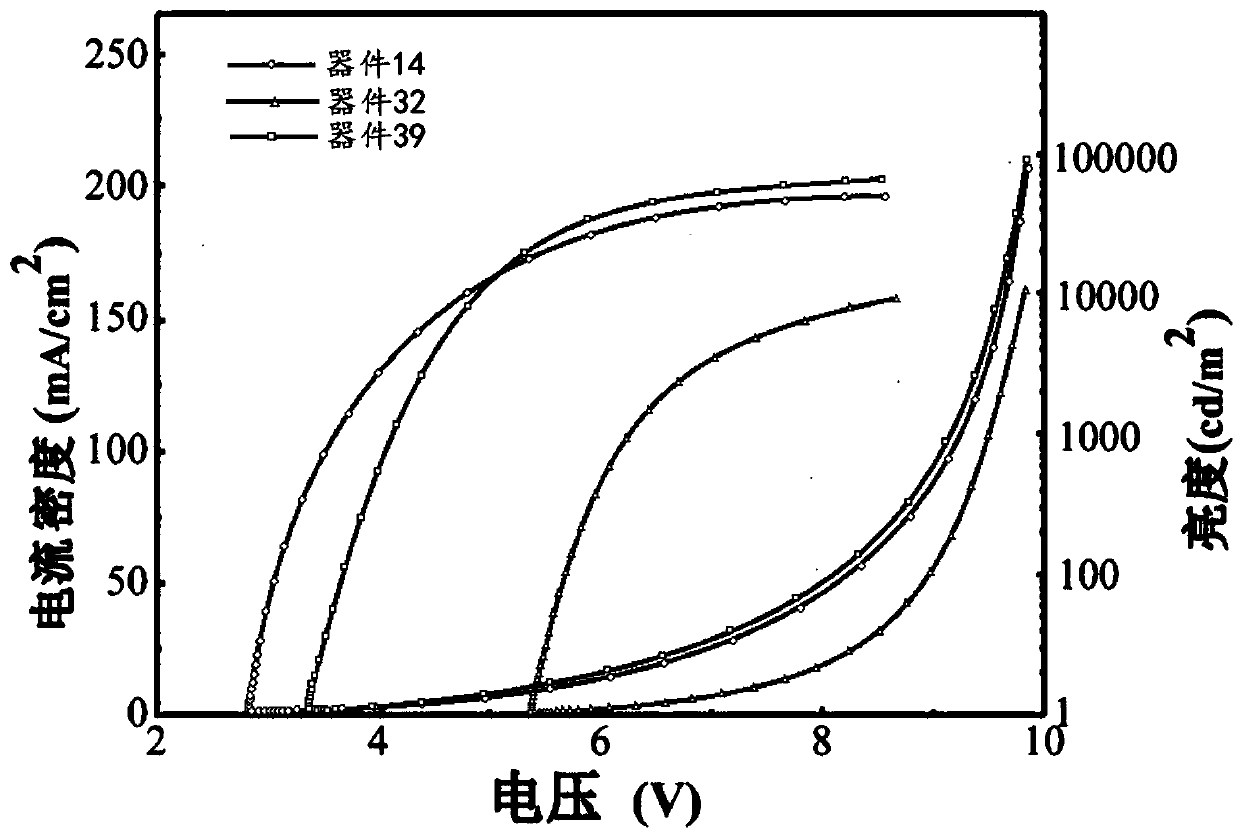
Fluorenone derivative, and preparation and application thereof
A technology of derivatives and fluorenones, applied in the field of fluorenone derivatives and their preparation and application, can solve the problems of incomplete removal of long-wave emission, oxygen quenching, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
[0051] In a 100mL three-necked flask, put 2-bromofluorenone (2.59g, 10mmol), [2,2'-binaphthyl]-6-ylboronic acid (2.98g, 10mmol), cesium carbonate (13.82g, 30mmol), Add 40mL tetrahydrofuran and 20mL water, under nitrogen atmosphere, add PdCl 2 (dppf)-CH 2 Cl 2 (0.16g, 0.2mmol), heated to 65°C and reacted for 6-24h, the liquid phase monitored the completion of the reaction, cooled to room temperature, filtered, concentrated, and separated by column chromatography to obtain 3.63g of the target compound with a yield of 84%.
[0052] Mass spectrometer MALDI-TOF-MS (m / z) = 432.5216, theoretical molecular weight: 432.5220; Anal.Calcd forC 33 h 20 (%): C 91.64, H 4.66, Found: C 91.65, H 4.65.
Embodiment 2
[0054]
[0055] Replace [2,2'-binaphthyl]-6-ylboronic acid (2.98g, 10mmol) with (10-phenylanthracene-9-yl)boronic acid (2.98g, 10mmol), and other basic The same method was used to prepare the target compound in the above formula (the molar ratio of the reactants and the reaction conditions were the same), and 3.46 g of the target compound could be obtained with a yield of 80%. Mass spectrometer MALDI-TOF-MS (m / z) = 432.5218, theoretical molecular weight: 432.5220; Anal.Calcd for C 33 h 20 (%): C 91.64, H 4.66, Found: C 91.64, H 4.65.
Embodiment 3
[0057]
[0058] Replace [2,2'-binaphthyl]-6-ylboronic acid (2.98g, 10mmol) with fluoranthene-3-ylboronic acid (2.46g, 10mmol), and prepare the others in the same manner as in Example 1 The target compound in the formula (the molar ratio of reactants and the same reaction conditions) can obtain 2.93 g of the target compound with a yield of 77%. Mass spectrometer MALDI-TOF-MS (m / z) = 380.4460, theoretical molecular weight: 380.4460; Anal.Calcd for C 29 h 16 (%): C 91.56, H 4.24, Found: C 91.54, H 4.25.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com