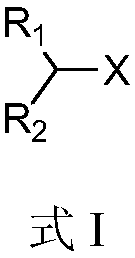
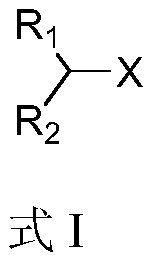
Alkyl-halide boron esterification reaction method free from transition metal catalysis
A technology of alkyl halides and alkyl borates, applied in the field of organic synthesis, can solve the problems of difficult handling, high cost, and complexity of the reaction, and achieve the effects of wide functional group compatibility and considerable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
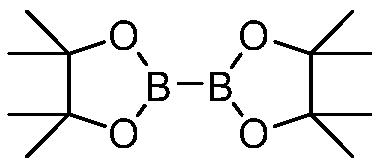
[0034] In the glove box, add t-BuOLi (0.75 mmol, 1.5 equivalents, 60.1 mg) to a vial equipped with a stirring bar, B 2 pin 2 (1.5mmol, 3 equivalents, 380.9mg), 0.85mL solvent methanol, 10 μL H 2 O, iodo-n-octane (0.5 mmol). The capped vial was removed from the glove box, and the reaction mixture was stirred at 50 °C for 48 hours. After cooling to room temperature, the reaction mixture was transferred to a 100 mL flask through methanol, and then a small amount of silica gel was added thereto. After the solvent was removed in vacuo, the residue was poured on a silica gel column, and purified by column chromatography. The developing solvent was a mixed solution of petroleum ether / ethyl acetate with a volume ratio of 50:1 to 30:1 to obtain the desired product. Octylboronic acid pinacol ester, yield 83%. The reaction was carried out in the same procedure using 0.5 mmol n-octane bromide, and the yield was 66%.
Embodiment 2
[0036] In the glove box, add t-BuOLi (1 mmol, 2 equivalents, 80.1 mg), B 2 (neop) 2 (Neopentyl glycol diboronate, 2mmol, 4 equivalents, 451.8mg), 0.85mL solvent methanol, 10 μL H 2 O, iodo-n-octane (0.5 mmol). The capped vial was removed from the glove box, and the reaction mixture was stirred at 50°C for 48 hours. After cooling to room temperature, the reaction mixture was transferred to a 100 mL flask through methanol, and then a small amount of silica gel was added thereto. After the solvent was removed in vacuo, the residue was poured on a silica gel column and purified by column chromatography. The developing solvent was a mixed solution of petroleum ether / ethyl acetate with a volume ratio of 30:1 to obtain the desired product n-octylboronic acid. Pentylene glycol ester, yield 67%.
Embodiment 3
[0038] In the glove box, add t-BuOLi (1 mmol, 2 equivalents, 80.1 mg), B 2 pin 2 (2mmol, 4 equivalents, 507.9mg), 0.85mL solvent methanol, 10 μL H 2 O, iodomethane (0.5 mmol). The capped vial was removed from the glove box, and the reaction mixture was stirred at 50 °C for 48 hours. After being cooled to room temperature, the reaction mixture was transferred to the test tube by methanol, a certain mass of internal standard n-decane was added, diluted with ethyl acetate, and the yield of the product methylboronic acid pinacol ester was measured by GC-fid method as 30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com