Oil-soluble chitosan derivative and preparation method and application thereof
A chitosan derivative and oil-soluble technology is applied in the field of oil-soluble chitosan derivatives and its preparation, which can solve the problems of reducing the adsorption of metal ions, the difficulty of chitosan molecules having both oil-solubility and adsorption properties, and the like. The effect of improving solubility, good solubility and dispersibility, and good maintenance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
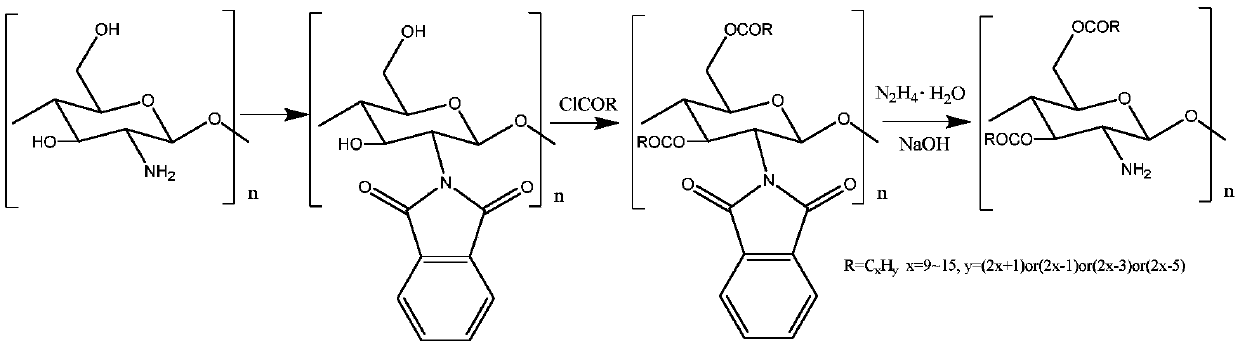
[0026] A preparation method of O-lauric acid acylated chitosan. Add chitosan to a certain amount of N,N-dimethylformamide with a volume ratio of 10% water, record the quality of chitosan as 1 part, add 3 parts of phthalic acid to react, and make the reaction at 100 °C under normal pressure React for 5 hours, filter and wash after the reaction to obtain the intermediate product; then, add the intermediate product and 1 part of lauric acid chloride to the isopropanol solvent for reaction, and stir the reaction at 70°C for 2 hours under normal pressure; then the amount of the substance used is Hydrazine monohydrate of 1.5 times of phthalic acid and concentration are 1mol / L sodium hydroxide aqueous solution (being 10 times of chitosan in terms of mass fractions) to remove phthaloyl group, and product is used after freeze-drying O-lauric acid acylated chitosan was obtained after washing with distilled water and freeze-drying again.
Embodiment 2
[0028] A preparation method of O-myristic acid acylated chitosan. Add chitosan to a certain amount of N,N-dimethylformamide with a volume ratio of 20% water, record the mass of chitosan as 1 part, add 3 parts of phthalic acid to react, and make it under normal pressure at 110 °C React for 4 hours, filter and wash the intermediate product after the reaction is completed; then, add the intermediate product and 3 parts of myristic acid chloride into isopropanol solvent for reaction, stir and react for 5 hours at 60°C under normal pressure; then use the amount of substance Hydrazine monohydrate 2 times that of phthalic acid and a concentration of 1mol / L sodium hydroxide aqueous solution (8 times that of chitosan in parts by mass) remove phthaloyl groups, and the product is freeze-dried After washing with distilled water and freeze-drying again, O-myristic acid acylated chitosan was obtained.
Embodiment 3
[0030]A preparation method of O-palmitic acid acylated chitosan. Add chitosan to a certain amount of N,N-dimethylformamide with a water volume ratio of 15%, record the quality of chitosan as 1 part, add 3 parts of phthalic acid to react, and make the reaction at 150 ° C under normal pressure React for 2 hours, filter and wash after the reaction to obtain the intermediate product; then, add the intermediate product and 2 parts of palmitic acid chloride to the isopropanol solvent for reaction, stir and react at 80°C under normal pressure for 1 hour; subsequently, the amount of the substance used is The hydrazine monohydrate of 1 time of phthalic acid and the concentration are 1mol / L sodium hydroxide aqueous solution (being 1 time of chitosan in terms of mass parts) to remove phthaloyl group, and the product is through after freeze-drying After washing with distilled water and freeze-drying again, O-palmitic acid acylated chitosan was obtained.
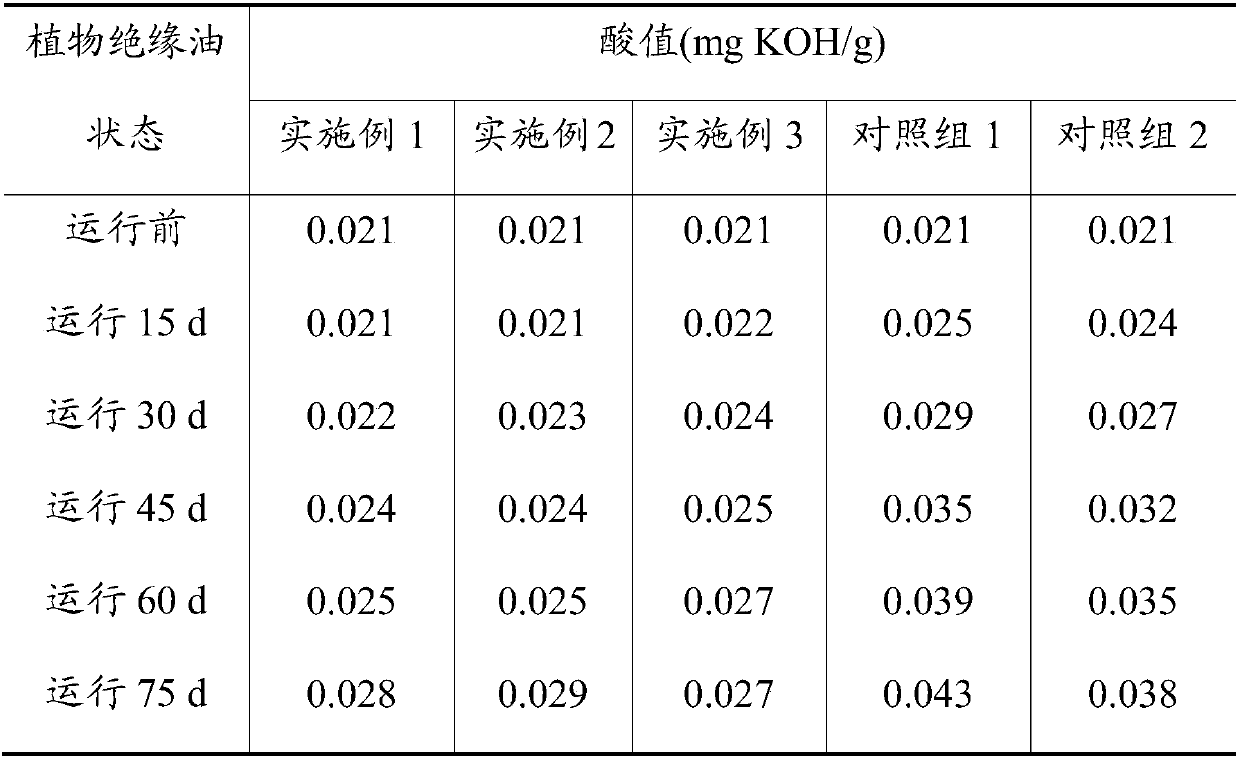
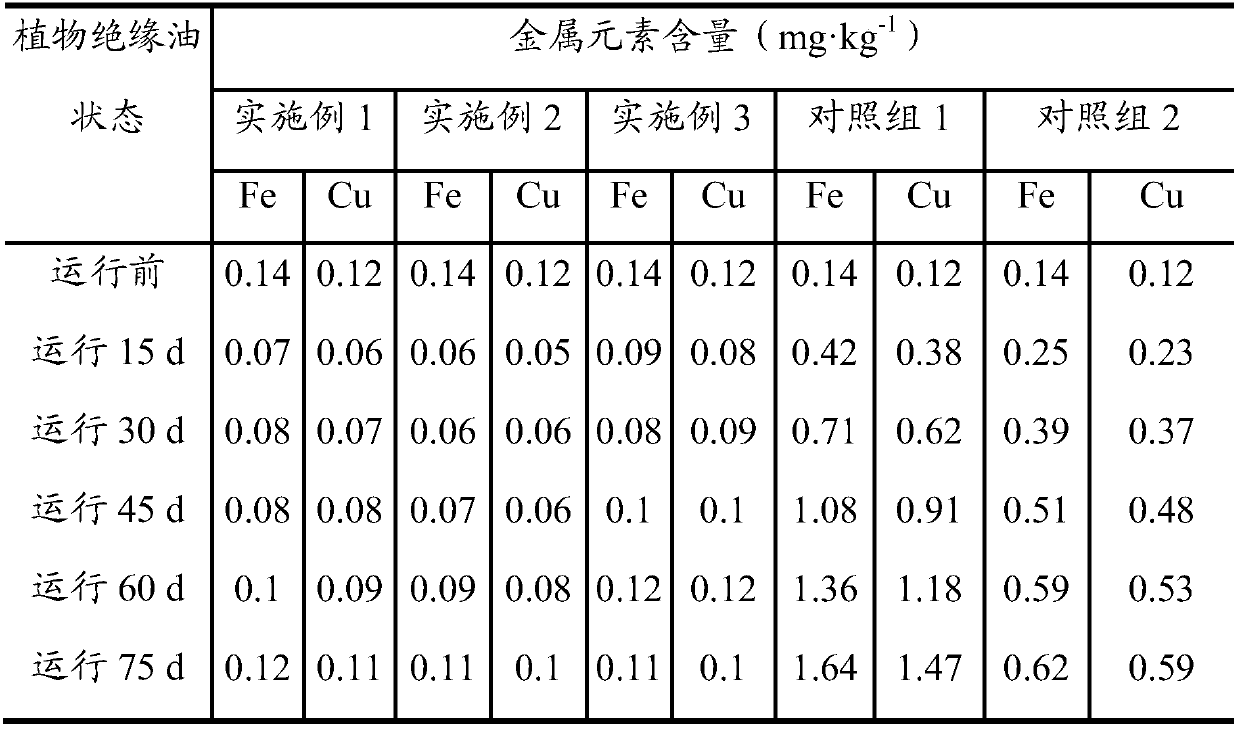
[0031] Get the prepared O-acylat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com