Application of tri (alkylamino) phosphonic compound, lithium ion battery, electrolyte and electrolyte additive
An electrolyte additive and lithium-ion battery technology, applied in the field of batteries, can solve problems affecting battery performance and safety, corrosion of Al positive electrode collectors, thermal instability of lithium hexafluorophosphate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] 1) Preparation of electrolyte
[0047] The electrolyte solution of embodiment 1~9 and comparative example 1~4 is prepared according to the following method:
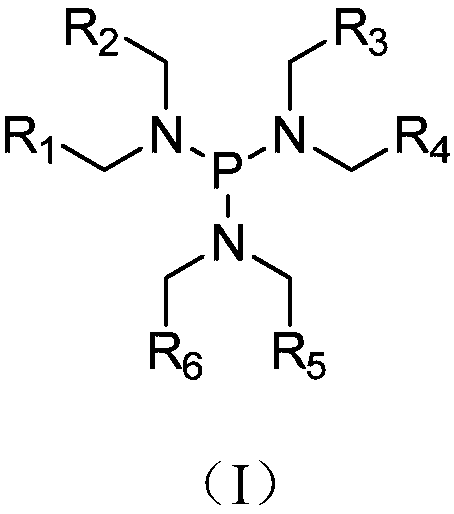
[0048] Mix ethylene carbonate (EC), diethyl carbonate (DEC) and ethyl methyl carbonate (EMC) in a mass ratio of EC:DEC:EMC=3:2:5, and then add bis(fluorosulfonyl)acyl Lithium imide (LiFSI) to a molar concentration of 0.8mol / L, the additives include additive A, additive B and additive C, wherein additive A is a tri(alkylamino)phosphine compound having a structure such as general formula (I). The type and content of additives in the electrolyte of the embodiment and the comparative example, the R in the additive A 1 ~R 6 The specific groups of are shown in Table 1, wherein the proportion of additives is the proportion of the total weight of the electrolyte.
[0049] Table 1 Embodiment 1~9 and the additive of comparative example 1~4 and content thereof
[0050]
[0051]
[0052] 2) Preparation of positive e...
Embodiment 2
[0074] Example 2, Examples 6-7 Compared with Examples 8-9, Example 2, Examples 6-7 have less corrosion effect on the positive current collector, and the battery capacity retention rates at 25°C and 45°C are higher .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com