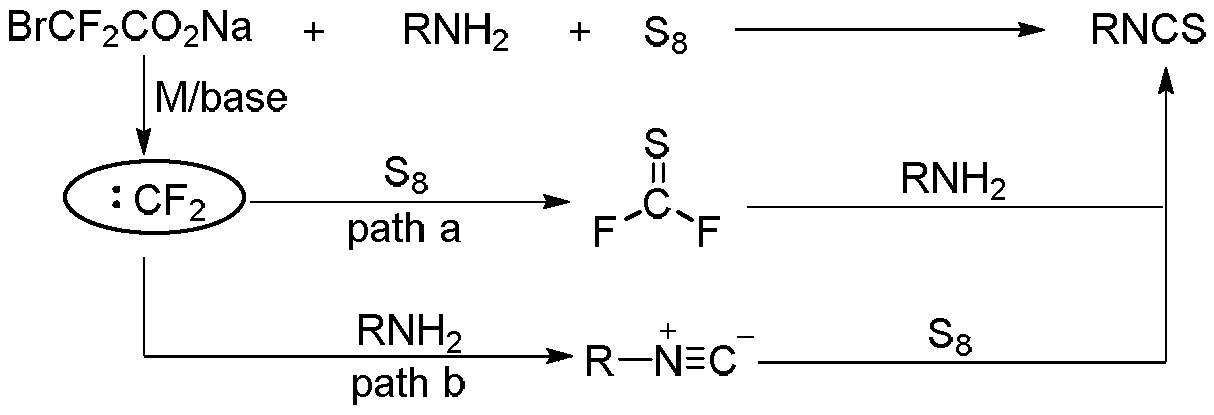
Isothiocyanate synthesized by three components and preparation method of isothiocyanate
An isothiocyanate and three-component technology, applied in the field of three-component synthetic isothiocyanate and its preparation, can solve the problems of limited practicability and applicability, long reaction time, cumbersome processing, etc. Ensuring the health of operators, strong substrate universality, and simplifying the effect of process engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0033] Specific embodiment one: with 28.6 milligrams (0.2mmol) 2-naphthylamine (1.0 equivalent), 1.9 milligrams (0.01mmol) cuprous iodide (5% equivalent), 59.2 milligrams (0.3mmol) sodium difluorobromoacetate (1.5 equivalent), 51.2 mg (0.2 mmol) S 8 (1.0 equiv), 84.9 mg (0.004 mmol) of potassium phosphate (2% equiv), was added to 2 mL of solvent acetonitrile. React at 100°C for 12 hours, cool down after the reaction, filter the reaction liquid to obtain the filtrate, then perform rotary evaporation on the filtrate, remove the solvent to obtain the residue, and use silica gel column chromatography to elute the residue with petroleum ether as the eluent , collected the effluent, detected by TLC, combined the effluent containing the product, distilled off the solvent with a rotary evaporator, and dried in vacuo to obtain 32.2 mg of 2-naphthalene isothiocyanate as a white solid with a yield of 87%. m.p.116-118℃. 1 H NMR (400MHz, CDCl 3 )δ7.83-7.80 (m, 2H), 7.77 (d, J = 8.0Hz, 1...
specific Embodiment 2
[0034] Specific embodiment two: with 28.6 milligrams (0.2mmol) 1-naphthylamine (1.0 equivalent), 1.9 milligrams (0.01mmol) cuprous iodide (5% equivalent), 59.2 milligrams (0.3mmol) sodium difluorobromoacetate (1.5 equivalent), 51.2 mg (0.2 mmol) S 8 (1.0 equiv), 84.9 mg (0.004 mmol) of potassium phosphate (2% equiv), was added to 2 mL of solvent acetonitrile. React at 100°C for 12 hours, cool down after the reaction, filter the reaction liquid to obtain the filtrate, then perform rotary evaporation on the filtrate, remove the solvent to obtain the residue, and use silica gel column chromatography to elute the residue with petroleum ether as the eluent , collected the effluent, detected by TLC, combined the effluent containing the product, distilled off the solvent with a rotary evaporator, and dried in vacuo to obtain 30.0 mg of 1-naphthalene isothiocyanate as a white solid with a yield of 81%. m.p.53-55℃; 1 H NMR (400MHz, CDCl 3 )δ8.10(d, J=8.0Hz, 1H), 7.87(d, J=8.0Hz, 1H)...
specific Embodiment 3
[0035] Specific example three: with 18.6 milligrams (0.2mmol) aniline (1.0 equivalent), 1.9 milligrams (0.01mmol) cuprous iodide (5% equivalent), 59.2 milligrams (0.3mmol) sodium difluorobromoacetate (1.5 equivalent), 51.2 mg (0.2 mmol) S 8 (1.0 equiv), 84.9 mg (0.004 mmol) of potassium phosphate (2% equiv), was added to 2 mL of solvent acetonitrile. React at 100°C for 12 hours, cool down after the reaction, filter the reaction liquid to obtain the filtrate, then perform rotary evaporation on the filtrate, remove the solvent to obtain the residue, and use silica gel column chromatography to elute the residue with petroleum ether as the eluent , collected the effluent, detected by TLC, combined the effluent containing the product, distilled off the solvent with a rotary evaporator, and dried in vacuo to obtain 22.4 mg of phenyl isothiocyanate, with a yield of 83%. 1 HNMR (400MHz, CDCl 3 )δ7.40(t, J=7.4Hz, 2H), 7.33(t, J=7.4HZ, 1H), 7.29-7.26(m, 2H); 13 CNMR (125MHz, CDCl 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com